Research
The Hinton laboratory at Vanderbilt has four areas of focus.
1. Molecular mechanisms of insulin action
We focus on elucidating insulin-mediated molecular mechanisms that regulate cristae dynamics and those that regulate molecular transfer between and morphological changes of the mitochondria and the ER. These can change during pathophysiological states, such as aging, diabetes, and cardiovascular disease. The laboratory heavily utilizes serial block-face scanning electron microscopy, time-lapse confocal microscopy, proximity ligation assay, and transmission electron microscopy quantification to answer these seminal questions.
2. Regulation of blood pressure, baroreflex sensitivity, and stroke in the brain
The goal of our research is to identify the novel neural circuits, neurotransmitters, and intracellular molecules in the brain that are critical for blood pressure, baroreflex sensitivity, and stroke. The findings of this research will identify rational targets for development of therapeutic strategies for hypertension. Specifically, we plan to generate unique mouse models using Cre-Lox recombination with genes of interest (e.g., nuclear transcription factors, ERα/β, SRC1-3, mitochondrial gene OPA-1, and MICOS complex genes associated with cristae dynamics) manipulated in specific populations of neurons at the site of interest (i.e., medial amygdala and paraventricular nucleus). These models will be used to establish the physiological relevance of specific neural networks in the regulation of blood pressure and stroke.
Hinton, A. O., Jr, He, Y., Xia, Y., Xu, P., Yang, Y., Saito, K., Wang, C., Yan, X., Shu, G., Henderson, A., Clegg, D. J., Khan, S. A., Reynolds, C., Wu, Q., Tong, Q., & Xu, Y. (2016). Estrogen Receptor-α in the Medial Amygdala Prevents Stress-Induced Elevations in Blood Pressure in Females. Hypertension (Dallas, Tex.: 1979), 67(6), 1321–1330. https://doi.org/10.1161/HYPERTENSIONAHA.116.07175
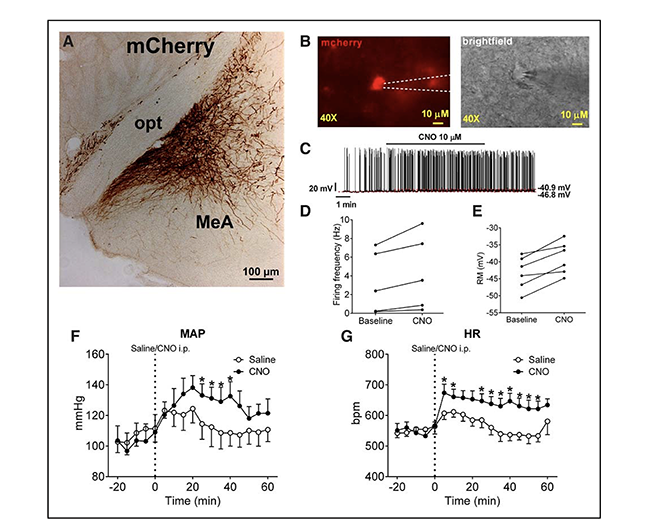
Figure: Effects of selective activation of medial amygdala (MeA) SIM1 neurons. A, mCherry immunoreactivity in the MeA of SIM-Cre mice following stereotactic infection with AAV-hM3Dq-mCherry. B, Electrophysiological recording from mCherry-labeled MeA SIM1 neurons in the brain slice from a SIM1-Cre mouse stereotactically infected with AAV-hM3Dq-mCherry. Fluorescence images of mCherry-labeled hM3Dq and brightfield images. Scale bars, 10 μm. C, Representative action potential traces in mCherry-labeled MeA SIM1 neurons in response to bath application of clozapine-N-oxide (CNO; 10 μmol/L). Firing frequency (D) and resting membrane potential (E) of each neuron at baseline and after CNO treatment. Temporal changes in mean arterial pressure (F) and heart rate (G) in AAV-hM3Dq-mCherry–infected SIM1-Cre mice receiving intraperitoneal injection of saline or CNO (3 mg/kg). Data are presented as mean ± standard error of the mean (SEM); n=7 per group. *P<0.05 in two-way analysis of variance followed by post hocSidak tests.
3. Method Building for Electron Microscopy Techniques
Utilization of electron microscopy (EM) in pathology diagnosis continues to be an important future avenue to explore as technology continues to advance. In the 1950s, a focus of EM utility on mitochondria began and since then there have been increased advances in diagnostic EM of biopsies. The most prominent way to characterize tissue by biopsies is to use H&E and TEM staining, which allows for one to look morphologically at gross histology, but only in a 2D plane. Given these techniques are in a 2D plane, they cannot give information regarding the 3D dynamics of mitochondria. As a result, most changes in the field are based on key changes in TEM, which limits the changes in dynamics that can be used to inform the medical community about when they encounter a biopsy that may be unique. Current advances in the field such as Focus Ion Beam-Scanning EM (FIB-SEM) and Serial Block Face-Scanning EM (SBF-SEM), which enable 3D reconstruction, are extensive and require proper techniques for sample preparation, machine operation, and quantitative computer analysis. Currently, we have developed protocols for the proper measurements of the mitochondrial connectome in 3D. Our methods seek to standardize the field while offering reproducible and clear protocols.
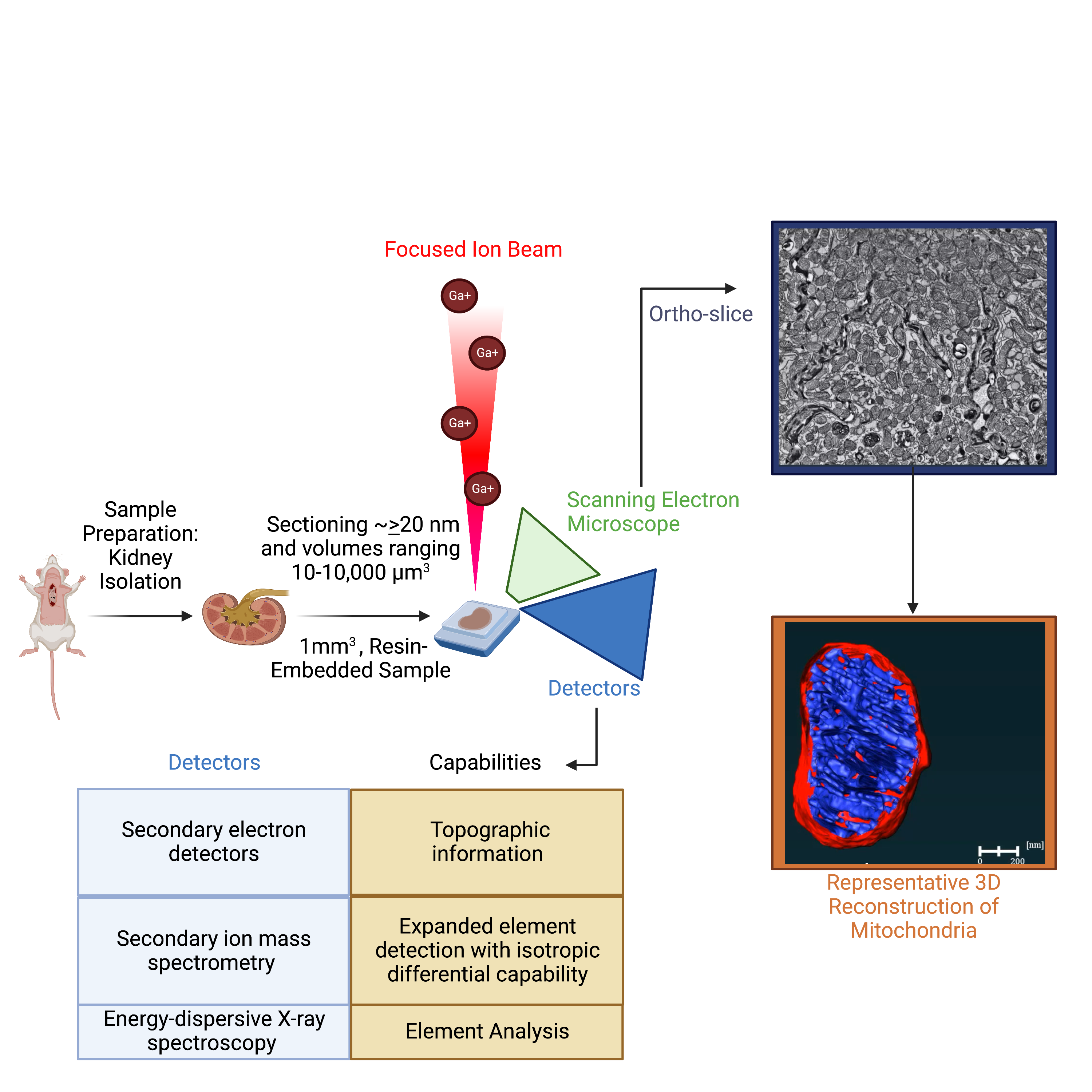
Figure: Schematic of focused ion beam scanning electron microscopy. Full explanation: Marshall, A. G., Damo, S. M., & Hinton, A., Jr (2023). Revisiting focused ion beam scanning electron microscopy. Trends in biochemical sciences, 48(6), 585–586. https://doi.org/10.1016/j.tibs.2023.02.005.
4. Investigating the Hallmarks of Aging – Mitochondrial Dysfunction
We perform the generation of knockout mice models and have successfully utilized 3D reconstruction to analyze the effects of aging. Our recent contributions have elucidated the impact of oxidative stress on mitochondrial bioenergetics in obesity, cardiovascular disease, and diabetes and the identification of novel regulation of mitochondrial integrity in the heart and skeletal muscle across aging in mouse models. Our studies have focused on elucidating insulin-mediated molecular mechanisms that regulate cristae dynamics and elucidate molecular mechanisms that regulate molecule transfer and morphology changes between the mitochondria. Our research program aims to broadly characterize mitochondrial 3D structural changes across aging states and correlate them with functional changes.
Video: Depiction of 2-year-old murine cardiac tissue mitochondria 3D reconstruction. Full results available: Vue, Z., Neikirk, K., Vang, L., Garza-Lopez, E., Christensen, T. A., Shao, J., Lam, J., Beasley, H. K., Marshall, A. G., Crabtree, A., Anudokem, J., Jr, Rodriguez, B., Kirk, B., Bacevac, S., Barongan, T., Shao, B., Stephens, D. C., Kabugi, K., Koh, H. J., Koh, A., … Hinton, A., Jr (2023). Three-dimensional mitochondria reconstructions of murine cardiac muscle changes in size across aging. American journal of physiology. Heart and circulatory physiology, 325(5), H965–H982. https://doi.org/10.1152/ajpheart.00202.2023.