Photosystem I Research
Photosystem I-Based Biohybrid Solar Energy Conversion
Photosynthesis is the process by which plants, algae and cyanobacteria convert solar energy into chemical energy. At the heart of this process are two protein complexes known as Photosystem I and Photosystem II. In this project, we isolate Photosystem I protein complex from spinach leaves and interface it with electrochemical mediators, electron/hole transport materials and electrodes (such as metals, semimetals and semiconductors). Through studying these protein-material interactions, we further our understanding of Photosystem I as a macromolecular photosensitizer. Our lab collaborates with the Jennings lab at the Chemical and Biomolecular Engineering department here at Vanderbilt University. Our collaborative research efforts aim to increase direct charge transfer between individual PSI protein molecules and reduce resistive losses associated with indirect charge transfer.
Current Work
-
Interfacing PSI with Carbon Nanomaterials:
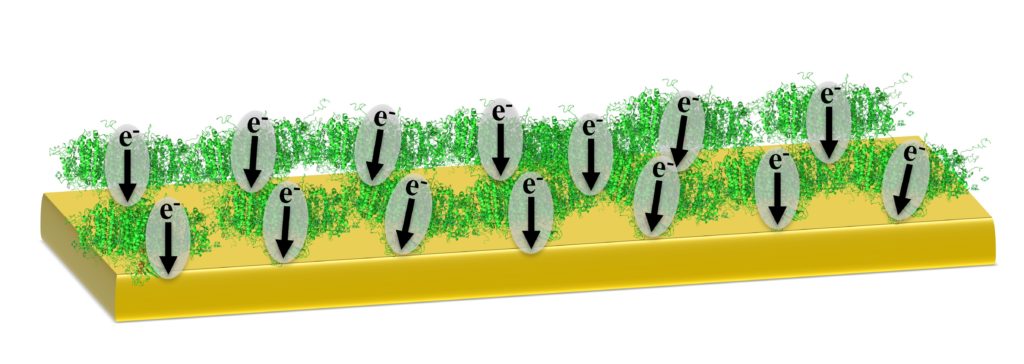
While PSI is arguably the most efficient solar energy converter on Earth, researchers have yet to approach its potential for biohybrid solar energy conversion. Interfacing PSI’s active sites (P700 and Fb) with nanoscale components that enable easier access for electron transfer would provide a tremendous leap in the solar conversion performance of PSI-based biohybrid systems. For this reason, we have synthesized and characterized a variety of organic nanocarbon materials and implemented them into our biohybrid devices. Examples of carbon interfaces currently being studied include doped carbon nanoparticles, or “carbon dots”, functionalized multiwalled carbon nanotube modified electrodes, and low cost, high surface area carbon paper electrodes.
-
Incorporating PSI into Conductive Three-Dimensional Frameworks:
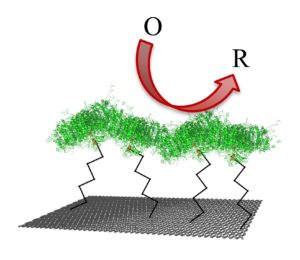
Interfacing PSI with three-dimensional frameworks such as nanostructured/nanoporous electrodes and/or conductive polymers provides a method for increasing areal protein loading. Increasing the electrochemically active surface area of an electrode positively impacts both direct and mediated electron transfer pathways, which leads to a boost in the generated photocurrent. Similarly, entrapping PSI into conductive polymer films enhance charge shuttling processes from individual proteins. Our group has successfully integrated PSI with conductive polymers such as polyaniline (PANI), polypyrrole, polyviologens and poly(3,4-ethylenedioxythiophene) as well as high surface area electrodes such as nanoporous gold leaf and nanostructured gold and ITO electrodes. Our efforts in investigating novel three-dimensional frameworks continue.
-
Development of Solid-State PSI-Based Photovoltaics:
Incorporation of PSI into all solid-state solar energy conversion architectures has several advantages. Elimination of the liquid electrolyte can significantly increase the operational stability and long-term durability of the solar cells. Not only the production of the reactive oxygen species in the solution damages the protein, but also the high salt content of the solution leads to the corrosion of the electrode. In addition, liquid electrolytes add unnecessary weight to the cell, may evaporate in hot environments or leak. For these reasons, our lab researches different ways to eliminate the need for these electrolytes. Solid electron and hole transport materials can replace the liquid electrolytes. These materials are chosen based on their energetic match with PSI’s two active sites, P700 and Fb.
-
Oriented PSI Monolayers for Aqueous-Organic Redox Flow Battery:
A new direction for the PSI project is to develop an oriented PSI membrane suspended at a liquid-liquid interface capable of transferring charge across the membrane. First, methods for the modification of PSI complexes to encourage specific orientation are being studied and developed. PSI orientation at liquid-liquid interface is determined largely by the functional groups on the protein complex surface and their interactions with the liquids. This design will allow for a uniform net electron transfer across the interface and may be used to develop a bio-inspired aqueous-organic redox flow battery charging system.