Bio Related Research
Biological Analyte Sensor Development for Pathological Study and Clinical Application
In the Cliffel Lab, we focus on developing sensors for biological purposes either for the purposes of studying a medical issue further, or to help identify a specific medicinal issue for clinical purposes. While all of our sensors are electrochemical, we use any potential avenue in order to achieve the desired specificity, including enzymes, ionophores, antibodies, and aptamers. Additionally, we collaborate with investigators in Biomedical Engineering, Obstetrics and Gynecology, Infectious Diseases/Pathology, and Orthopaedics.
Given the complexity of the human body, various problems can overlap, and a sensor can have more than one purpose, but these are the main projects that we have currently in our lab.
Current Work
-
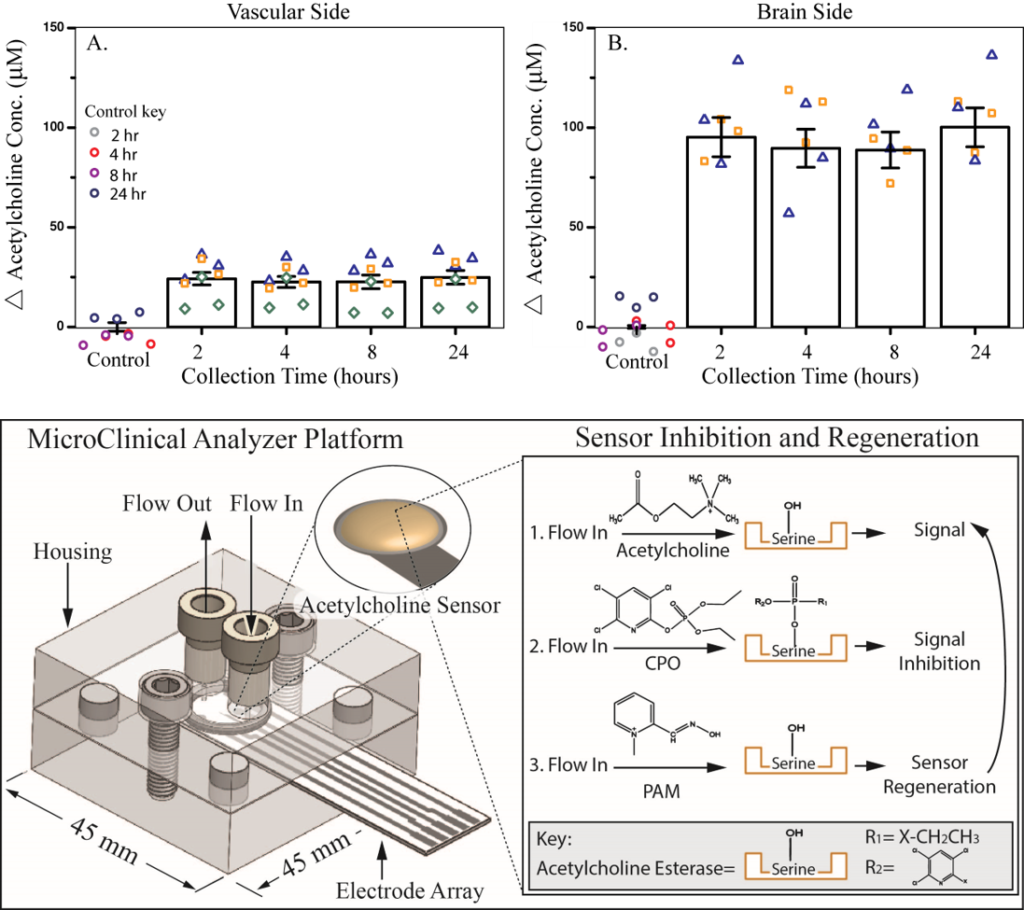
Neurovascular unit (NVU), an organotypic model of the blood-brain barrier (BBB) and the effects of Organophosphate (OP) compounds:
Organophosphate (OP) compounds, used throughout the agricultural industry as insecticides, are known to directly and irreparably alter brain function in humans. Exposure to OPs decreases acetylcholinesterase activity and leads to a buildup of acetylcholine, with chronic exposure to sub-lethal levels inducing neuropathy. This buildup of acetylcholine can be monitored through electrochemical methods to study the effects of OP toxicity. The microclinical analyzer (µCA), an in vitro microfluidic device allowing for electrochemical analysis using a screen-printed electrode, can be modified with enzymes to detect acetylcholine and other neurotransmitters. Using the µCA in combination with the neurovascular unit (NVU), an organotypic model of the blood-brain barrier (BBB), can provide a better understanding of the BBB forms, functions, and responds to insults. The NVU supports all the cell types necessary for proper BBB formation (endothelial cells, astrocytes, pericytes, and neurons) and provides the flow-created shear forces for mature tight junction formation. The µCA and NVU can be used to study the effects of chlorpyrifos and other toxins on neurotransmitter concentrations present across the BBB. Understanding the effects of these toxins on neurotoxicity can contributes to the assessment and treatment of chronic and acute exposure and inform policy decisions around their use.
-
Organotypic Cultures for Predictive Toxicology (Organs-on-Chips):
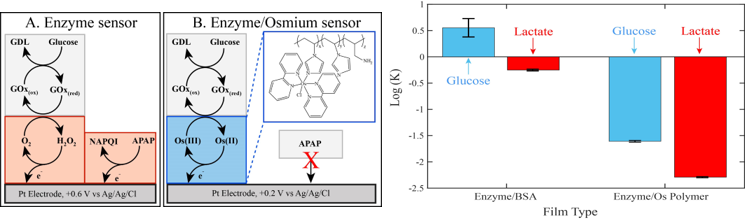
The failure of traditional tissue culture techniques to faithfully reproduce human microphysiological environments has been the impetus for the development of organs-on-chips (OoCs). These devices more accurately mimic physiological conditions and have allowed us to gain a better understanding of how various tissues, organs, and organ systems interact with one another and respond to chemical and physical perturbations. Key to gaining this insight is the ability to monitor cellular responses in a multiplexed and time-dependent fashion. We accomplish this using the microclinical analyzer (μCA), an electrochemical device capable of simultaneously measuring the concentration of various analytes in the effluent from OoCs. This research focuses on modifying the screen-printed electrodes used in conjunction with the μCA to customize the device for measurements downstream of an OoC. Specifically, there is work in fabricating amperometric enzymatic biosensors and potentiometric ionophore films for monitoring cholesterol, ammonium, calcium, and potassium, in addition to glucose, lactate, glutamate, dissolved oxygen, and pH which were measured in previous iterations of the device. Furthermore, a new sensor has been developed to remove electroactive interference in the signal by using an osmium-based redox polymer to mediate the electron transfer from an oxidase enzyme to the electrode. By taking advantage of this osmium polymer’s oxidation potential, a lower electrode bias was employed to avoid the bulk oxidation of an interferent, and therefore diminish interference. These sensors maintained excellent sensitivity, demonstrated increased selectivity of the analyte over the interferent, and exhibited longevity. Therefore, these low-interference tools provided a high-resolution platform for improved analyses in several potential applications including, toxicity studies on model organs, clinical studies, and in vivo studies on the brain. These approaches will ultimately allow the screening of a small library of drug candidates or environmental toxins for toxicity at a low cost and with accurate results.
-
Investigation of endometriosis and preterm birth utilizing Instrumented Fetal Membrane-on-a-Chip (IFMOC):
The first time the immune system can respond to a pathogen is in utero during infections of the fetal membrane. Infection involving the fetal membranes is extremely difficult to study in utero, both because of inaccessibility and the nature of the complicated interface of mother and child. Thus, studies of pregnancy-related conditions benefit from an in vitro model of the fetal membrane, i.e., a highly instrumented fetal membrane on a chip (IFMOC). Specifically, the overarching goal of this research project is to apply multidimensional analytical technologies and microfluidics engineering design to define immune response biosignatures of infection in the in vitro fetal membrane. Given these signatures, our ultimate long-range goal for this bench-to-bedside research program is to develop a simple, inexpensive, and robust lab-on-a-chip system that will permit accurate etiological diagnosis of infections early during the course of illness based on a fundamental understanding of the human systems biology of infectious diseases and will benefit from the recent advances in organ-on-chip microfluidics, optical, amperometric, and enzymatic sensors, and mass spectrometry. Our initial multianalyte sensor profiles are focused on cellular bioenergetics using glucose consumption and lactate production and oxidative burst by superoxide production measure by our microfabricated amperometric sensors as well as MIC-1 protein secretion by the quartz crystal microbalance; subsequently these signatures will be expanded with ion mobility-mass spectrometry (IM-MS).
-
Cytokine and Small Protein Sensor Development – Sepsis and IFMOC:
Cytokines are class of proteins that are small, but can still not cross the lipid bi-layer of the cell membrane which makes them ideal for cell signaling. Cell signaling is how our body communicates within itself, therefore a wide variety of biological processes and systems with which cytokines are relevant. Two of these biological examples are sepsis and the fetal membrane.
Sepsis is the result of a process known as acute phase response (APR) gone awry. Acute phase response (APR) is part of an immune defense that is activated when the body has detected a trauma or acute illness. The cytokine Interleukin-6 (IL-6) can be used to monitor and evaluate the severity of a patient’s APR. Post-operative patients or those following another sort of invasive trauma, the tracking of the patient’s IL-6 levels would allow the attending physicians to ensure that the patient is healing properly, and not approaching sepsis. If IL-6 levels were increasing, that would insinuate that the patient was still in APR and the body was not healing properly. This can give physicians the opportunity to intervene long before the patient has wide-spread inflammation, or multiple organ failure.
The cytokines Matrix Metalloproteinase-3 (MMP-3) and Matrix Metalloproteinase-9 (MMP-9) are known to be the enzymes responsible for breaking down the fetal membranes when it is time for birth to occur. What is still unclear is if or why these cytokines trigger early leading to preterm birth. Sensors detecting these cytokines for real-time analysis with the IFMOC would assist in the determination of their role in the breakdown of the fetal membrane, or potentially their triggering of other gestational issues.
We currently are fabricating duel sensor platforms for both static and flow and are evaluating two modes of specificity, antibodies and aptamers.