Research
Natural product total synthesis. We have maintained a longstanding interest in the development of synthetic strategies leading to the total synthesis of complex natural products. Historically, natural products present opportunities to address defined synthetic challenges presented in the novel structure of a natural product target. For example, we were the first and to date only group to complete the total synthesis of an unusual bicyclo[1.1.0]butane containing cyanobacterium metabolite (Angew Chem 2011) and bis-alkylpiperidine marine natural product (Angew Chem 2010). We have also developed methods and strategies to access complex natural products that demonstrate unique biological activity such as the apoptolidins (J Org Chem 2008) and angucycline antibiotics (J Am Chem Soc 1995 & 1998). These studies also serve as an excellent opportunity for the training of young scientists interested in the art and practice of chemical synthesis. Increasingly our motivation in the area of total synthesis is the biological study of synthetic products and derivatives. Natural products of current interest are shown below.
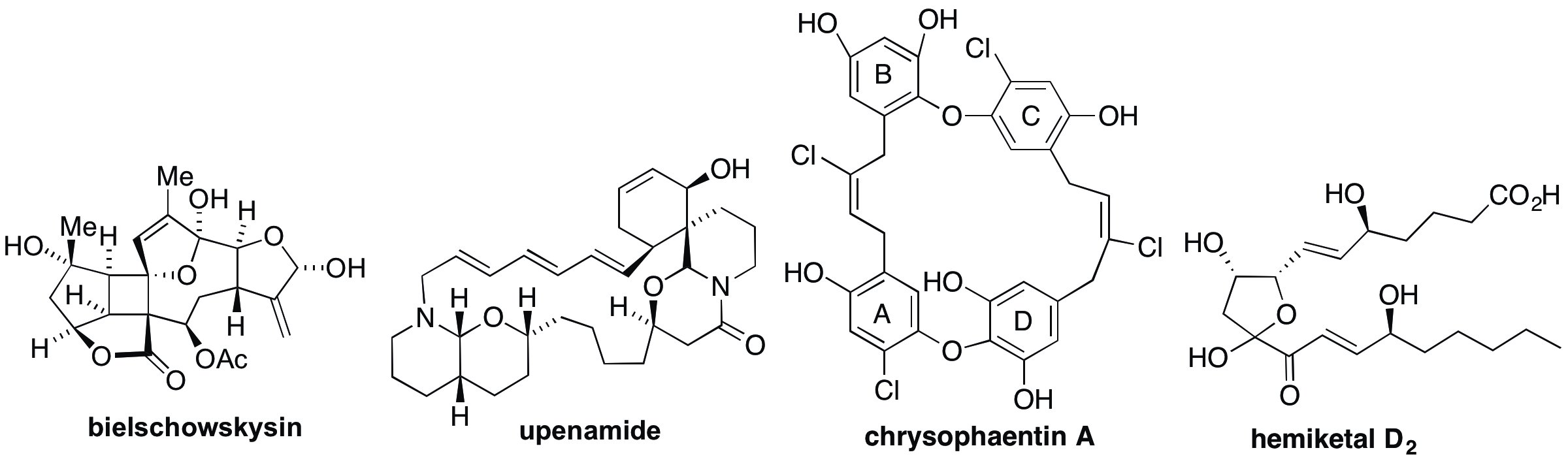
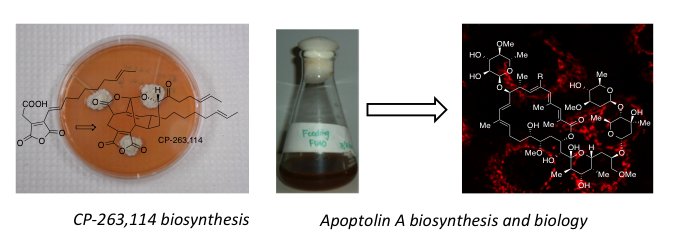
Biology and chemistry of natural products. Natural products are among the most structurally complex and privileged of the bioactive small molecules. Analysis of the sources of small molecule drugs approved between 1981 and 2006 indicates that over half of the new chemical entities declared in this timeframe were natural products or derived from natural products. However, from the perspective of medicinal chemistry, the complex molecular architecture of natural products continues to pose a significant challenge to access not only quantities of the parent structure but also analogs to establish structure-activity relationships (SAR). In addition to utilizing our skills in chemical synthesis to access and modify natural products we also attempt to harness the biosynthetic machinery to access complex natural products, sometimes in collaboration with groups with expertise the genetic modification of biosynthetic pathways. We were the first group to identify the biosynthesis of the complex nonadrides CP-225,917 and CP-263,114 and study the development of this biosynthetic dimerization in the lab. More recently, working with the Bachmann group we have developed fluorescent derivatives the cell selective cytotoxic agent apoptolidin and demonstrated its localization in the mitochondria. We are also one of two groups in the world that developed a chemical synthesis of the microbial thiol bacillithiol, now being used by a variety of investigators worldwide.
Chemical probes and pre-clinical lead development. Phenotypic and functional screens of compound libraries are currently being used by a variety of biomedical investigators in Cell and Developmental Biology, Microbiology and Immunology and Pharmacology. We provide medicinal chemistry support in lead development. In some cases we have optimized lead potency and DMPK properties allowing animal studies to provide preliminary validation of new targets for therapeutic development. Examples include Wnt signaling inhibitors for the treatment of cancer and GIRK selective activators that were advanced to animal studies validating their potential for the treatment of epilepsy. In these cases our role was primarily lead optimization using standard SAR studies. In collaboration with the Skaar group (Microbiology and Immunology) we have been studying small molecules that effect Staphylococcus aureus virulence and developing affinity probes to identify the cellular targets that lead to the observed bacterial phenotype.