Transcriptional control of synaptic specificity
The mechanism whereby neurons select specific synaptic partners is a long-standing and still largely unsolved problem in neurobiology (Fig. 1). Our approach to this question is motivated by the discovery that the C. elegans unc-4 gene controls the specificity of synaptic inputs to a particular class of ventral cord motor neurons (Fig. 2) (White et al., 1992). Molecular analysis revealed that unc-4 encodes a homeodomain transcription factor that is selectively expressed in VA motor neurons to prevent the adoption of synaptic inputs normally reserved for their VB sisters (Miller et al., 1992;Miller et al., 1995).
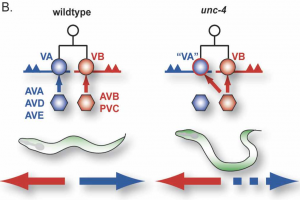
Backward locomotion is disrupted in unc-4 mutants in which VA motor neurons are miswired with inputs (gap junctions from AVB and chemical synapses from PVC) normally reserved for VB sisters.
UNC-4 interacts with UNC-37/Groucho to regulate synaptic choice in the motor circuit
This result was initially surprising because it suggested that synaptic specificity can be explicitly controlled by transcriptional regulation. To delineate the mechanism of this process, we utilized an Unc-4 modifier screen that detected interactions with allele-specific suppressor mutations in the unc-37 gene (Miller et al., 1993).
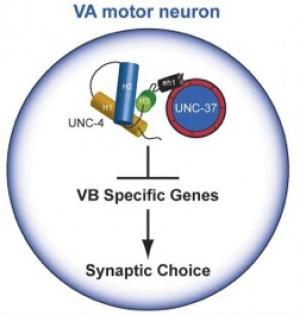
Our work revealed that unc-37 encodes the C. elegans homolog of Groucho, a highly conserved transcriptional co-repressor protein (Pflugrad et al., 1997) (Fig 2). The finding that UNC-4 function requires interaction with UNC-37/Groucho provided the first example of Groucho-dependent regulation of motor neuron fate and presaged the later discovery of the broad role of Groucho interactions with homeodomain proteins in the differentiation of spinal cord neurons (Muhr et al. 2001).
UNC-4 represses CEH-12/HB9 to specify synaptic inputs to VA motor neuron
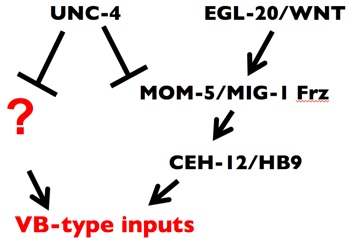
We proposed that UNC-4 functions with UNC-37/Groucho in VA motor neurons to prevent expression of VB genes that drive miswiring with VB-type inputs (Fig. 3) (Winnier et al., 1999; Lickteig, et al., 2001). To identify these genes, we have exploited genomic profiling strategies (Fox et al., 2005) designed to detect transcripts that are regulated by UNC-4 in VA class motor neurons (see Genomic Profiling). This approach revealed over 200 candidate UNC-4-regulated genes. We have confirmed that one of these targets, the HB9 homeodomain homolog, CEH-12, is normally restricted to VB motor neurons and that ectopic expression of ceh-12 in VAs is both necessary and sufficient to induce miswiring with VB-type inputs (Von Stetina et al., 2007).
 (Experiments testing other candidate UNC-4 target genes are underway.) The striking conservation of CEH-12/HB9 function in the determination of motor circuit fate in nematodes, insects, birds and mammals is indicative of an evolutionarily ancient role and therefore suggests that our findings in C. elegans may have revealed a primordial function for CEH-12/HB9 in motor neuron differentiation (Von Stetina, et al., 2006.pdf).
(Experiments testing other candidate UNC-4 target genes are underway.) The striking conservation of CEH-12/HB9 function in the determination of motor circuit fate in nematodes, insects, birds and mammals is indicative of an evolutionarily ancient role and therefore suggests that our findings in C. elegans may have revealed a primordial function for CEH-12/HB9 in motor neuron differentiation (Von Stetina, et al., 2006.pdf).
UNC-4 antagonizes a Wnt signaling pathway to specify motor neuron inputs
Although forced expression of CEH-12/HB9 is sufficient to induce miswiring of VA motor neurons throughout the C. elegans ventral nerve cord, ectopic CEH-12/HB9 is restricted to a subset of posterior VAs in unc-4 mutants (Von Stetina et al., 2007). We showed that CEH-12/HB9 expression in these posterior VA motor neurons depends on an EGL-20/Wnt signal from adjacent cells in the tail region. We propose that UNC-4 antagonizes a downstream canonical Wnt signaling pathway by negatively regulating the Frizzled receptors, MOM-5 and MIG-1 and thereby preventing VAs from responding to an available Wnt cue.
 Our results show that Wnt can direct the creation of both gap junction and chemical synaptic connections between specific neurons in the motor circuit. The widely observed occurrence of A/P Wnt gradients in animal phyla indicates that this mechanism may be evolutionarily ancient and thus deeply embedded in the genetic programs that govern the creation of complex spinal cord circuits (Schneider, Skelton, Von Stetina et al., 2012).
Our results show that Wnt can direct the creation of both gap junction and chemical synaptic connections between specific neurons in the motor circuit. The widely observed occurrence of A/P Wnt gradients in animal phyla indicates that this mechanism may be evolutionarily ancient and thus deeply embedded in the genetic programs that govern the creation of complex spinal cord circuits (Schneider, Skelton, Von Stetina et al., 2012).
UNC-4 regulates parallel downstream pathways to control synaptic choice
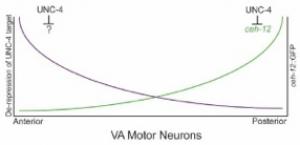 Our finding that CEH-12/HB9 expression is limited to posterior VAs in unc-4 mutants indicates that other UNC-4 target genes must drive the miswiring of anterior VAs. We have used a genetic strategy, based on our results with ceh-12, to search for these genes. ceh-12 loss-of-function mutations result in the restoration of backward locomotion to weak (“hypomorphic”) unc-4 mutants presumptively due to the restoration of VA-type inputs to posterior VAs (Von Stetina et al., 2007).
Our finding that CEH-12/HB9 expression is limited to posterior VAs in unc-4 mutants indicates that other UNC-4 target genes must drive the miswiring of anterior VAs. We have used a genetic strategy, based on our results with ceh-12, to search for these genes. ceh-12 loss-of-function mutations result in the restoration of backward locomotion to weak (“hypomorphic”) unc-4 mutants presumptively due to the restoration of VA-type inputs to posterior VAs (Von Stetina et al., 2007).
This finding suggests that loss-of-function mutations in unc-4 target genes that affect anterior VAs should also “suppress” the Unc-4 phenotype. An unbiased genetic screen for blr mutants (backward locomotion restored) has now revealed > 15 independent loci that suppress Unc-4. Preliminary genetic analysis has confirmed that at least three of these genes function in parallel to ceh-12 to regulate synaptic choice. We are now using whole genome sequencing to reveal the molecular identity of these blr genes.
Currently working on the Project…
Seth Taylor
Tyler Kennedy
Sierra Palumbos
Becca Collings
Rebecca McWhirter