Research
I am fascinated by the development, function and adaptability of the nervous system. My research focuses on the genetic mechanisms underlying coordinated movement, behavior and cognition. How does nervous system circuitry underlying behavior develop? How are nervous system circuits modified by experience? How do these mechanisms go awry in inherited neurological diseases and age-related neurological decline? These questions center around the common themes of information transfer and information storage in cells of the nervous system. My long-term focus has been on the intercellular synapses that establish and provide communication between nerve cells.
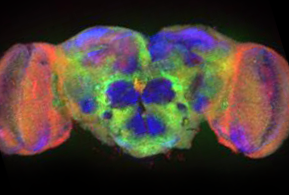
I use a genetic approach in Drosophila to tackle these questions. My laboratory uses a combination of forward genetics (direct screens for mutant phenotypes), reverse genetics (targeted mutation of identified genes) and functional genomics/proteomics (genome-wide biochemical assays). Our basic strategy is to generate mutants in genes essential for synapse development or function, and then to assay mutant phenotypes as a means to elucidate the normal function of gene products. Our primary target is genes involved in establishing synapses during embryonic development (synaptogenesis), genes that mediate communication during movement and behavior (neurotransmission), or genes that maintain the developmental potential of synapses throughout life (synaptic plasticity). Synaptic development involves specifying and constructing an intercellular communication link between two cells. This developmental program specifies synaptic partnerships and aligns a presynaptic signaling field (vesicle cycling) with the postsynaptic receptor field (ligand-gated ion channels). At maturity, the function of the synapse is to translate electrical information (action potentials) into chemical information (secreted neurotransmitters) and back again. This information transfer requires mechanisms to couple an action potential to the fusion of neurotransmitter vesicles, and receipt of the signal by the receptor field. Synaptic plasticity is the process whereby synaptic structure and/or function is altered is response to this information flow (i.e. amount of use), to modulate communication either transiently (learning) or permanently (memory).
An increasing interest in my laboratory is to develop genetic models of human neurological diseases linked to inherited synaptic dysfunction. One focus is Fragile X syndrome, the most common inherited neurological disease which results in cognitive impairment. Fragile X syndrome is caused by an arrest of synaptic development due to inappropriate translational regulation. We also study genetic models of progressive neurodegenerative diseases including Niemann-Pick C (NPC), Spastic Paraplegias and Parkinson’s Disease (PD). We are investigating the hypothesis that these diseases are caused by progressive loss of synaptic transmission, which is required to maintain neuronal viability.
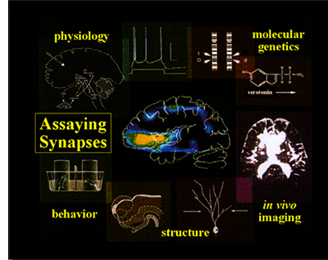
The approach in my lab to studying nervous system development, function and plasticity is multi-disciplinary and requires the marriage of many traditionally distinct fields. The work requires classical geneticists, molecular biologists, biochemists, developmental cell biologists, anatomists and electrophysiologists. In the long term, I hope that this work will lead to a greater understanding of neural network formation, mechanisms of integrated neuronal communication, higher brain functions including learning and memory, and the cure for synaptic dysfunction arising in inherited neurological diseases.