Protein Aging Mechanisms
Due to the unique physiology and biochemistry of the ocular lens, proteins in the lens nucleus (core) are as old as the animal in which they were synthesized. Thus, the lens contains abundant long-lived proteins (LLPs) and is an excellent model tissue in which to examine protein aging over decades of life.
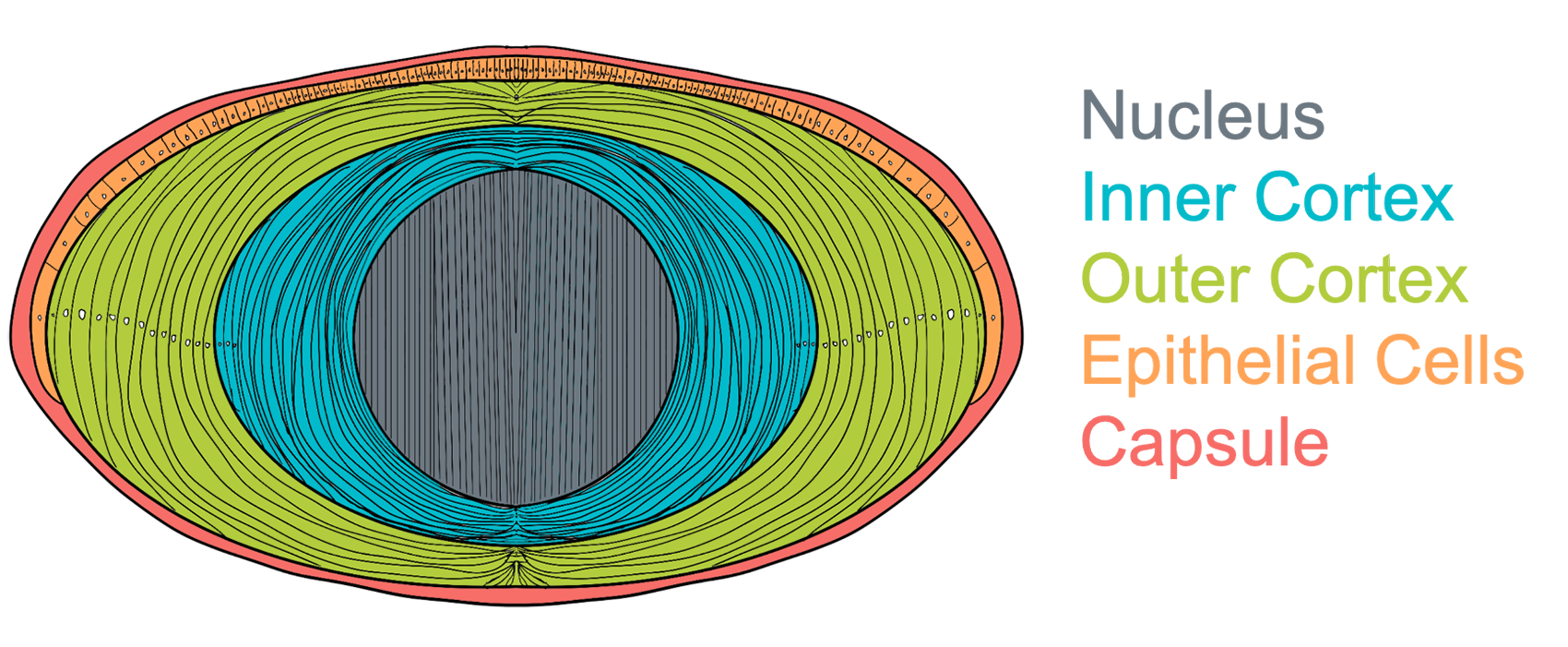
Cartoon diagram of human lens showing distinct spatial regions of varying age.
Typical age-related protein modifications include: deamidation, truncation, and crosslinking. The Schey lab uses spatially-resolved proteomics strategies to identify and quantify novel age-related protein modifications and seeks to identify such modifications in other long-lived proteins in tissues such as brain, lung, heart, cartilage, etc. We have identified at least five distinct endogenous protein crosslinking mechanisms.
Related References
Cantrell LS and Schey KL. Proteome Remodeling of the Eye Lens at 50 Years Identified with Data-Independent Acquisition. Mol. Cell. Proteom., 22:100453, 2023.
Paredes J, Wang Z, Schey KL. Dehydroalanine and Dehydrobutyrine in Aging and Cataractous Lenses Reveal Site-Specific Consequences of Spontaneous Protein Degradation. Front. Ophthalmol., 3:1241001, 2023.
Schey KL, Wang Z, Rose KL, Anderson DMG. Imaging Cataract-Specific Peptides in Human Lenses. Cells, 11:4042, 2022.
Cantrell LS and Schey KL. Data Independent Acquisition Mass Spectrometry of the Human Lens Enhances Spatiotemporal Measurement of Fiber Cell Aging. J Amer. Soc. Mass Spectrom., 32:2755-2765, 2021.
Giblin FJ, Anderson DMG, Han J, Rose KL, Wang Z, Schey KL. Acceleration of age-induced proteolysis in the guinea pig lens nucleus by in vivo exposure to hyperbaric oxygen: a mass spectrometry analysis. Exp. Eye Res., 210:108697, 2021.
Friedrich M, Wang Z, Schey KL, Truscott RJW. Spontaneous cleavage at Glu and Gln residues in long-lived proteins. ACS Chem. Biol., 16:2244-2254, 2021.
Friedrich MG, Wang Z, Schey KL, Truscott RJW. Spontaneous protein-protein crosslinking at glutamine and glutamic acid residues in long-lived proteins. Biochem J., 478(2): 327-339, 2021.
Cantrell LS, Schey KL. Proteomic Characterization of the Human Lens and Cataractogenesis. Exp. Rev. Proteomics, 18:119-135, 2021.
Schey KL, Wang Z, Friedrich M, Truscott RJW. New Insights into the Mechanisms of Age-Related Protein-Protein Crosslinking in the Human Lens. Exp Eye Res, 209:108679, 2021.
Schey KL, Wang Z, Friedrich MG, Garland DL, Truscott RJW. Spatiotemporal changes in the human lens proteome: critical insights into long-lived proteins. Progress in Retinal and Eye Research, 78:100802, 2020.
Friedrich MG, Wang Z, Schey KL, Truscott RJW. Mechanism of protein cleavage at asparagine leading to protein-protein crosslinks. Biochem. J. 476:3817-3834, 2019.
Wang Z, Friedrich MG, Truscott RW, Schey KL. Cleavage C-terminal to Asp Leads to Covalent Crosslinking of Long-Lived Human Proteins. Biochim Biophys Acta – Proteins and Proteomics, 1867:831-839, 2019.
Friedrich MG, Wang Z, Schey KL, Truscott RJW. Spontaneous Cross-linking of Proteins at Aspartate and Asparagine Residues is Mediated via a Succinimide Intermediate. Biochem. J., 475:3189-3200, 2018.
Wang Z and Schey KL. Quantification of Thioether-Linked Glutathione Modifications in Human Lens Proteins. Exp. Eye Res. 175:83-89, 2018.
Friedrich MG, Wang Z, Schey KL, Truscott RJW. DehydroalanylGly, a New Post Translational Modification Resulting from the Breakdown of Glutathione. Biochim. Biophys. Acta 1862:907-913, 2018.
Friedrich MG, Wang Z, Oakley A, Schey KL, Truscott RJW. Hotspots of Age-Related Protein Degradation. The Importance of Neighbouring Residues for the Formation of Non-Disulfide Crosslinks Derived from Cysteine. Biochem J. 474:2475-2487, 2017.
Truscott RJW, Schey KL, Friedrich MG. Old Proteins: A Field in its Infancy. Trends in Biochemical Sciences, 41:654-664, 2016.
Slavi N, Wang Z, Harvey L, Schey KL, Srinivas M. Identification and Functional Assessment of Age-Dependent Truncations to Cx46 and Cx50 in the Human Lens. Invest. Ophthalmol. Vis. Sci. 57:5714-5722, 2016.
Wang Z, Lyons B, Truscott RJW, Schey KL. Human Protein Aging: Modification and Crosslinking through Dehydroalanine and Dehydrobutyrine Intermediates. Aging Cell, 13:226-234, 2014.