Research Interests
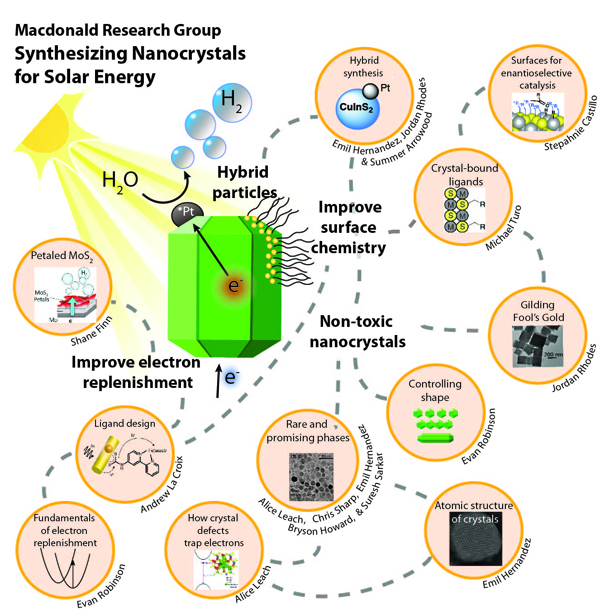
As the world is threatened by climate change, there is increasing pressure to develop new green technologies and decrease our carbon footprint. One such technology is developing hydrogen fuel using solar power. While solar energy technology is more commonly used to directly power homes and businesses with electricity, solar-to-fuel technology has the possibility of replacing fossil fuels for heating and transportation –use of these fuels represents the one of the largest causes for increased greenhouse gases. One of the most attractive alternative fuels is hydrogen, which can be obtained from splitting water molecules its components, hydrogen and oxygen. The hydrogen can then be used to power cars, heating plants and other combustible engines with only water as its by-product. However, it is energy intensive to create hydrogen, so by leveraging solar energy to make it, the whole process becomes carbon neutral.
Using sunlight to split water was first reported in the 1970s, but despite decades of effort, the efficiencies have remained stubbornly low. The goal is to create a solar panel that can easily and efficiently produce hydrogen by splitting water molecules. A major problem with these panels is the materials, or crystals, used in them. The large, micron-sized, crystals of light-capturing material are generally not very efficient.
The process of solar energy capture begins when light is absorbed in the crystal, and an electron is excited. The excited electron must travel to the surface of the crystal to react with water molecules and make hydrogen. Often the surface of the crystal has other particles on it to help facilitate this reaction of changing water into hydrogen. While a micron may seem small to us, to an electron, a micron is the equivalent of someone swimming to a surface of a lake hundreds of feet deep. It is very easy for excited electrons on that journey to become trapped by flaws in the crystal, or simply lose their energy due to vibrations of the atoms around them. This vastly decreases the efficiency of these crystals to turn light intohydrogen. If these crystals could be even smaller, i.e. nano-sized, the possibility of this happening greatly reduces and the efficiency of the material improves.
As a postdoctoral fellow in the laboratory of Professor Uri Banin at the Hebrew University of Jerusalem (2008-2010), I was a part of research that developed the synthesis of cadmium sulfide nanocrystals with attached metal dots. Such nanocrystal designs can produce hydrogen with efficiencies of near 100% under some conditions. This very exciting result brings us much closer to realizing an efficient solar-to-fuel technology, but hurdles remain. The nanocrystals are sometimes unstable, and begin to degrade over time in these reactions. This happens because the nanocrystals must be refreshed with donor electrons from the surrounding molecules in solution. This process of donating is thousands of times slower than the speed at which electrons are leaving the crystals to make hydrogen, thus degrading the crystal quality. In addition, to date we use sacrificial molecules to replenish electrons that are consumed in the process. If we wish to have a true solar energy program based on water splitting, we need to make hydrogen and oxygen using the nanocrystals rather than using non-renewable molecules. Lastly, the light absorbing materials used are often made of cadmium sulfide which contains highly toxic cadmium and therefore not ideal for mass production.
My research program’s goals are multi-fold:
1) Develop new chemistry at the surfaces of nanocrystals to prevent them from degrading and make the process of electron replenishment faster and renewable;
2) Explore using new less-toxic light-absorbing materials like copper sulfide and it’s related family of structures;
3) lastly to develop methods to carefully grow small catalytic domains on the surfaces of the nanocrystals (“hybrid” nanocrystals), so we can grow them in the specific places to maximize the speed creating hydrogen gas.
Not only will this research potentially make a significant difference in industry and the world’s efforts to reduce greenhouse gases, but we develop a better understanding of the atomic detail on how the nanocrystals form and how molecules attach to their surfaces. The skills we learn here can also be applied to nanocrystals for eventual use in solar cells, fluorescent biological tags and LED lighting.
I believe in a collaborative approach to advance our ambitious goals which is why our team has been working extensively with theoretical physicists at Vanderbilt and Lawrence Berkley National Lab to model the nanocrystal surfaces and defects in the structure that trap electrons, electron microscopists at Oak Ridge National Laboratory to obtain atomic resolution images of the nanocrystals, and spectroscopists at Vanderbilt and at The Hebrew University of Jerusalem in Israel to measure the intimate details about how our new nanocrystals absorb light and what happens to that energy. We leverage Vanderbilt’s particular resources by heavily utilizing the characterization tools at the Vanderbilt Institute for Nanoscale Science and Engineering (VINSE) such as the electron microscopes.