We have openings for new grad students and postdocs to work on cutting edge research projects in cancer biology, human embryonic stem cell biology, and epithelial homeostasis. Our students go on to outstanding postdoctoral positions and many have become successful independent investigators. Postdoctoral fellows in the Macara lab have frequently achieved tenure track faculty positions at R1 institutions.
Open projects include: (1) Understanding collective behaviors of epithelial cells including cancer cell extrusion and cell intercalation, using synthetic biology technologies; (2) Determining mechanisms of mesoderm induction and epithelial to mesenchymal transition in human embryonic stem cells; (3) Investigating the mechanisms by which Mammary Ductal Carcinoma in Situ (DCIS) transitions to Invasive Ductal Carcinoma (IDC).
The Macara laboratory is a diverse group of scientists, with past and present students and postdocs from France, Russia, the UK, Ecuador, Bangladesh, India and Canada, as well as the US. We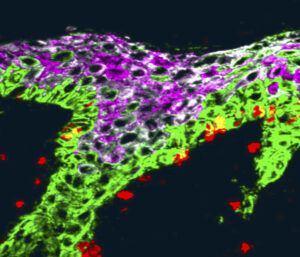 strongly support racial, gender, and geographic diversity and inclusivity.
strongly support racial, gender, and geographic diversity and inclusivity.
We are excited by fundamental questions about epithelial behavior in development, in homeostasis, and during cancer initiation. Embryonic stem cells are epithelial, and undergo an epithelial-mesenchymal transition (EMT) when they begin to differentiate into certain lineages. Loic Fort, a postdoc in the lab, has discovered two remarkable events that occur during human iPSCs or ESCs differentiation along the mesoderm/cardiomyocyte lineage. First, there is a rapid wave of apoptosis, followed at 49 hours post-induction of differentation by an abrupt EMT. Astonishingly, the apoptosis is essential for the stem cells to respond to the WNT signal that switches on differentiation. This work was published in Nature Cell Biology (2022).
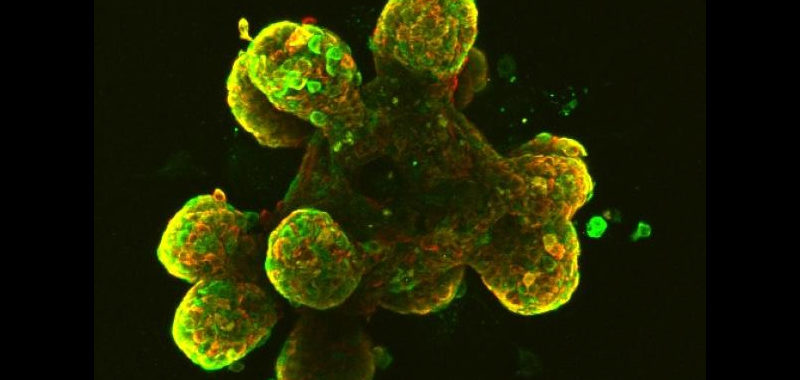
Most human cancers arise from epithelial tissues. We use the mouse mammary gland and human organoids as model systems (Dev Cell 2020). Transgenic mice provide powerful tools to probe stem cell function, breast cancer initiation mechanisms, and responses to injury. We are particularly interested in the transition from mammary Ductal Carcinoma in Situ (DCIS) to Invasive Ductal Carcinoma (IDC), and in the mechanisms by which tumor cells escape the mammary ducts into the surrounding stroma.
We also use cell culture to determine how epithelial cells interact with one another and with cancer cells. We are using synthetic biology approaches to track how polarity proteins are delivered to the correct location on the plasma membrane. We have also discovered that mammary epithelial cells can intercalate into confluent monolayers, and used computational modeling to show that this process is essential to ductal elongation during development (Dev Cell 2023).
We have developed genome-wide CRISPR screens to identify novel genes involved in epithelial homeostasis (eLife 2020). We also endogenously tag proteins involved in epithelial cell polarity, using CRISPR gene-editing, so as to track these proteins with single molecule sensitivity. We employ multi-channel TIRFM and near-TIRF microscopy, which provides unprecedented spatial and temporal resolution of protein complex dynamics (Nature Commun 2018).
INTERESTED IN A POSTDOCTORAL POSITION OR PhD RESEARCH IN THE LAB? – CONTACT IAN MACARA AT: ian.g.macara@vanderbilt.edu.