Ongoing Research
The focus of the Jacobson lab is on understanding how ion channels of islet-cells and dorsal root ganglion (DRG) sensory neurons influence the pathogenesis of diabetes and pain. Ion channels control Ca2+ entry into islet-cells, which is required for hormone secretion that regulates blood glucose homeostasis. In DRG sensory neurons electrical excitability and Ca2+ influx regulates pain perception. Calcium influx into islets and DRG neurons becomes perturbed in patients with diabetes and chronic pain respectively. However, the mechanism(s) responsible for perturbed Ca2+ homeostasis in these cells have not been defined. Therefore, the goal of the Jacobson lab is to determine if ion channels that modulate islet hormone secretion and DRG neuron nociception can be utilized as therapeutic targets for treating diabetes and pain.
The Function of Two-Pore-Domain Potassium Channels
Potassium channels play a significant role in regulating islet hormone secretion and nociception by tuning Ca2+ entry into islets and DRG neurons. Islet cell and DRG neuron potassium channels regulate the membrane potential of these cells, which controls Ca2+ entry through voltage-dependent Ca2+ channels. However, many of the potassium currents of islets and DRG neurons have yet to be examined in the context of diabetes and chronic pain. The Jacobson lab has determined that the two-pore-domain potassium (K2P) channels TASK-1 and TALK-1 are highly expressed in pancreatic islet-cells (JCI Insight, PMID: 34032641; Diabetes, PMID: 28550109; Molecular Endocrinology, PMID: 25849724); whereas the K2P channel TREK-2 is highly expressed in DRG sensory neurons (ACS Chemical Neuroscience, PMID: 27805811). Furthermore, the Jacobson team has discovered that K2P channels are expressed on the endoplasmic reticulum (ER) membrane where they regulate Ca2+ release from the ER (Science Signaling, PMID: 28928238; Molecular Metabolism, PMID: 29402588). Therefore, the Jacobson lab is testing the hypothesis that K2P channels control electrical excitability, ER Ca2+ release, and ER exodosis in Islet-cells and DRG neurons, thus, modulating hormone secretion and pain sensation. Results from these projects will significantly expand our understanding of islet hormone secretion and pain perception. Moreover, these projects will illuminate if K2P channels can be utilized as therapeutic targets for treating diabetes and chronic pain.
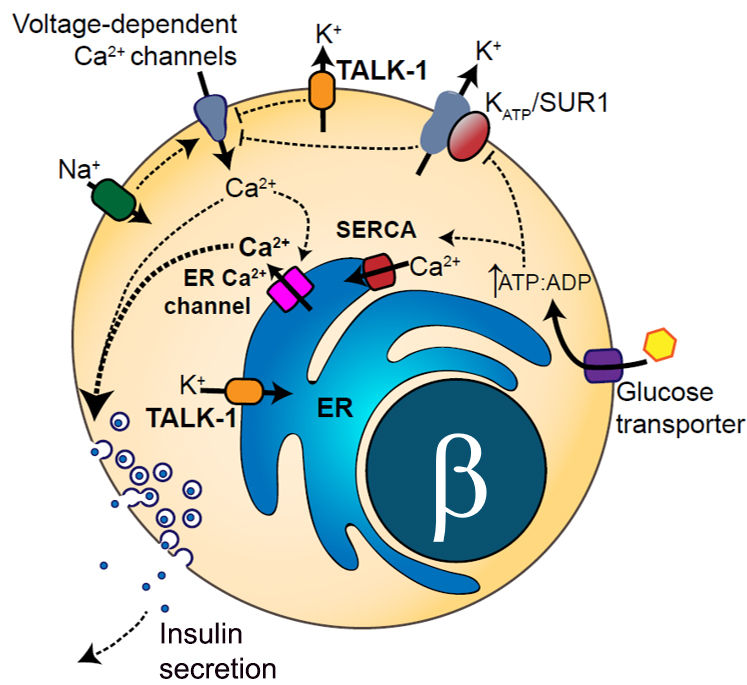
Regulation of Islet Electrical Activity and Hormone Secretion by Paracrine Signaling and the Local Microenvironment
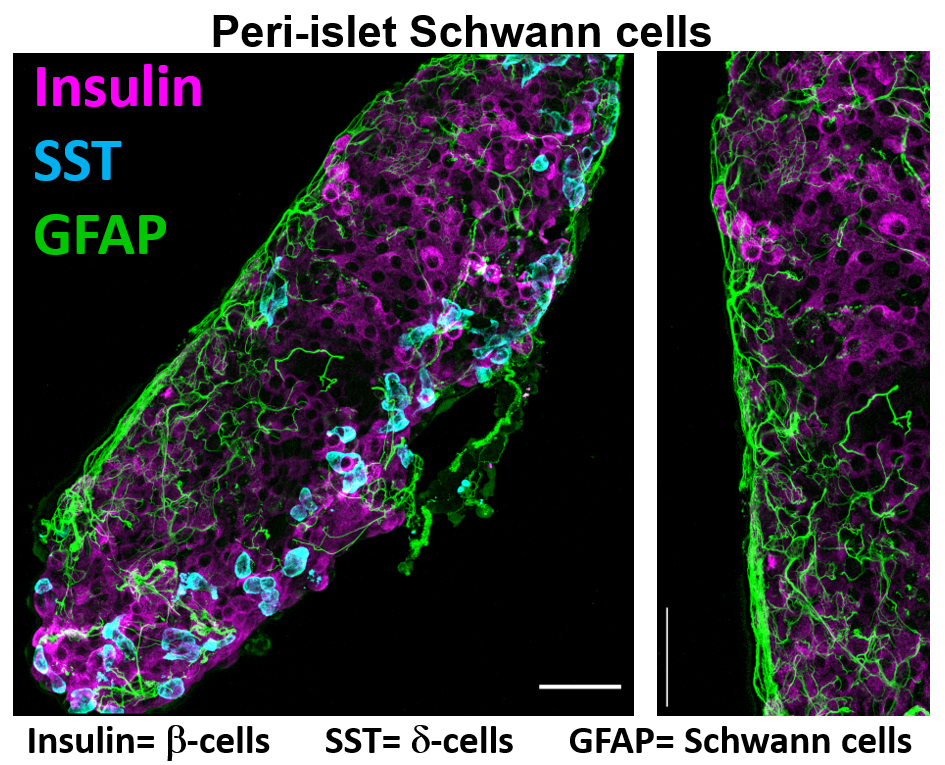
The Jacobson lab is also investigating how paracrine signaling within the pancreatic islet microenvironment influences Ca2+ homeostasis and hormone secretion under physiological and diabetic conditions. These studies are assessing ionotropic glutamate receptor (iGluR) control of islet glucagon secretion and insulin secretion. One part of this project is to determine how iGluR interacting proteins influence glutamatergic control of islet-cell Ca2+ influx and hormone secretion. Furthermore, this project is assessing how peri-islet Schwann cells (pSCs) that surround the islet control neuronal inputs into the pancreatic islet as well as their ability to buffer islet glutamate levels. Finally this project is assessing how stress induced pSC gliosis impacts islet function. Results from these projects will illuminate how glutamate signaling controls islet function and if pSC signals can be used to stimulate islet function and prevent beta cell destruction during the pathogenesis of diabetes.
Dissertation projects are tailored around the research foci of the Jacobson lab that stimulate your curiosity and organized in manner that enables success (e.g. multiple publications and job opportunities within 5 years).