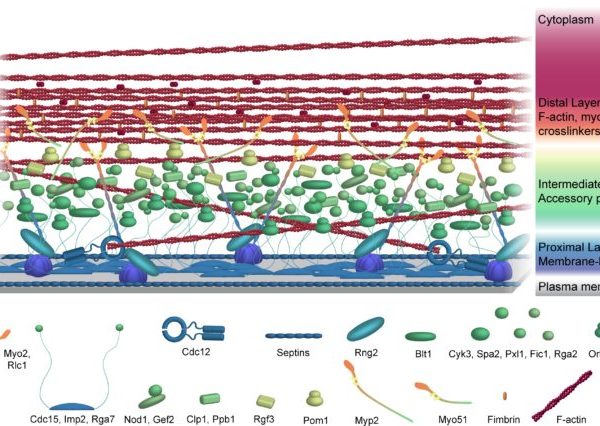
II. F-BAR proteins
Uniting characteristics of the F-BAR protein family are the presence of a membrane-binding F- BAR domain and a role in linking membranes to the F-actin cytoskeleton. Like many F-BAR domains, Cdc15’s F-BAR domain binds the membrane and oligomerizes. Membrane binding and oligomerization are essential for the stable association of the CR at the cell equator as mutations that abolish membrane binding or oligomerization cause CR sliding during constriction. F-BAR oligomerization is also critical for physiological function of human F-BAR proteins Fer and RhoGAP4, demonstrating its broad importance to F-BAR proteins (McDonald et al., 2015).
Unexpectedly, we discovered that Cdc15, Fer, and RhoGAP4 plus 4 additional human F-BAR proteins do not bend or tubulate membranes. Even more remarkably, studies with Imp2, a Cdc15 paralogue, revealed that although it tubulates membranes in vivo, this activity is dispensable for its cellular function (McDonald et al., 2016). These studies identified a sub-group of F-BAR proteins without membrane tubulation activity and provided strong evidence in support of the hypothesis that membrane binding and oligomerization—not tubulation activity—are the vital properties of F-BAR proteins in vivo (McDonald et al., 2016).
Predictably, Cdc15 functions are carefully regulated. Like Cdc12, Cdc15 is regulated by phosphorylation. Upon entry into mitosis, Cdc15 dephosphorylation triggers a conformational change that results in its oligomerization and allows interaction with binding partners. Using mass spectrometry, a large number of phosphorylation sites were identified (>30) and mutation of these sites to non-phosphorylatable residues resulted in precocious Cdc15 oligomerization and the formation of pre-ring assemblies of cytokinetic proteins. This in turn led to defects in CR construction, constriction, and disassembly (Roberts-Galbraith et al., 2010).
We have now found that the Pom1 is one of the kinases that directly phosphorylates Cdc15 on 22 sites. Pom1 is a DYRK kinase that is tip localized to inhibit CR formation at cell tips partly through the direct phosphorylation of Cdc15. By phosphorylating Cdc15, Pom1 inhibits Cdc15’s membrane-binding activity as well as its ability to bind Pxl1 at cells tips. However, Pom1 phosphorylation of Cdc15 does not regulate the ability of Cdc15 and Fic1 to interact. In either wildtype or mid1∆ cells, we propose that Cdc15 can bind membrane and Pxl1 to stabilize the CR only where Pom1 is not active (see figure below) (Bhattacharjee, Mangione, et al., 2019).
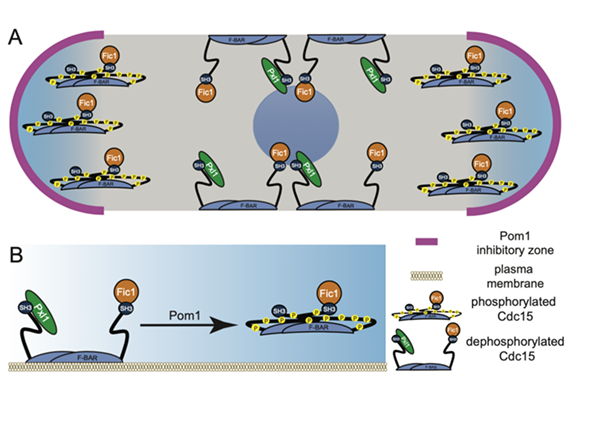
Models of Pom1 inhibition of Cdc15 function.
A) Model of Pom1 inhibition of Cdc15 membrane binding at cell tips in a single interphase cell with the nucleus in the middle.
B) Molecular model of Pom1 inhibition of Cdc15 membrane binding.
We are currently identifying additional protein kinases and phosphatases that contribute to Cdc15 phosphoregulation and determining how they affect the ability of the F-BAR domain to oligomerize and bind membranes and protein partners. We are also using mathematical modeling to understand whether different signaling inputs have different outputs in terms of cytokinesis control via Cdc15 scaffolding function.