Welcome to the Zhou Lab!
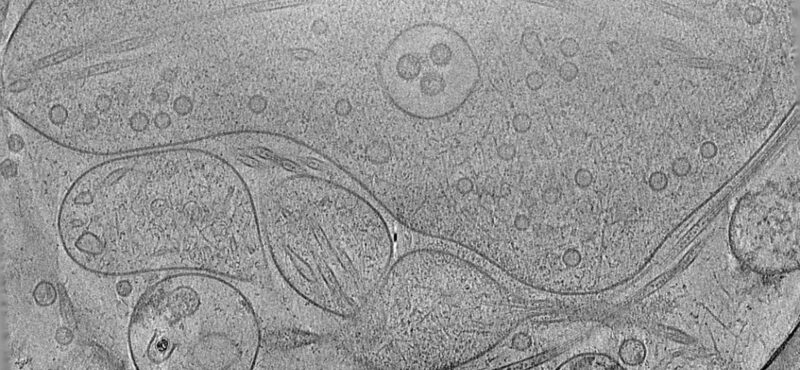
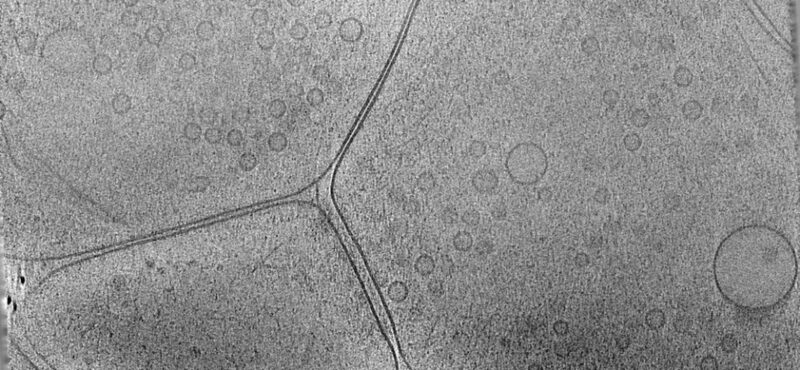
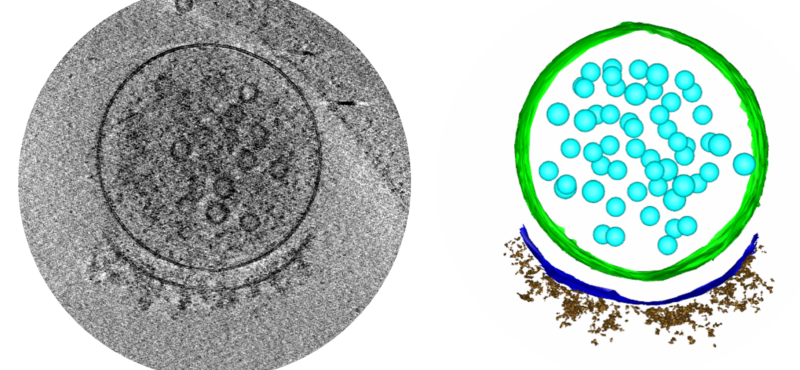
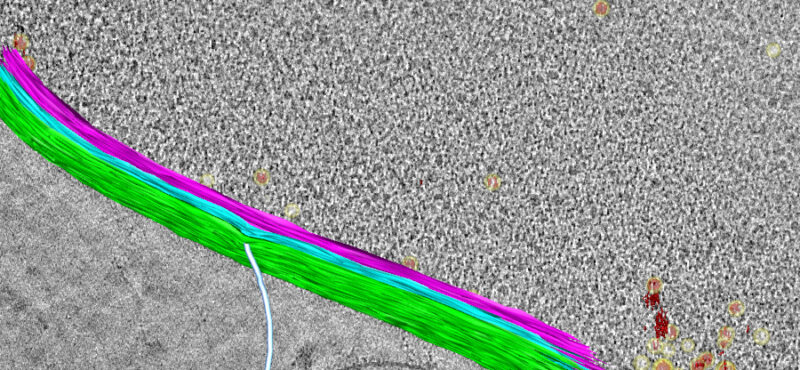
To elucidate fundamental mechanisms of cellular functions and diseases, we must understand the spatial and temporal organization of multi-protein assemblies and their interactions with other cellular components at the molecule level. Neuronal chemical synapses are typical examples of such nanoscale structures. Efficient synaptic transmission depends on the trans-cellular alignment and high-order organization of nanoscale supramolecular assemblies spanning the synaptic cleft. The nanoscale, activity-dependent reorganization of key synaptic proteins, including neurotransmitter receptors, is widely known as a mechanism of synaptic plasticity. Synaptic plasticity is essential for perception, decision making, learning, and memory formation. Additionally, major components of these synaptic protein assemblies are associated with neurodevelopmental disorders and other mental illnesses. Our long-term goal is to address foundational questions on synaptic transmission and plasticity using the emerging framework of macromolecular assemblies, thereby contributing to the development of pharmacological therapeutics for neurodevelopmental disorders.