Transport
All molecules larger than ~30kDa (or ~8nm) must display a signal to a transport receptor prior to moving through the NPC. Binding of this signal by the receptor generates a transport-competent complex that moves through the pore via a series of low-affinity interactions with phenylalanine-glycine (FG) polypeptide repeats. One third of the nucleoporins (Nups) contain these regions of FG repeats. Known collectively as FG-Nups, these proteins are found in distinct distributions within the NPC: some are found exclusively on the cytoplasmic face of the NPC, some are only on the nuclear face of the NPC, and others are distributed symmetrically through the pore’s interior. We have established a vast collection of tools for systematically dissecting the individual character of these different FG domains in Saccharomyces cerevisiae, and have uncovered important discrete functions for many FG-Nups. Interestingly, we have shown that each of the FG-Nups play distinct roles in different transport pathways through the NPC. However, many mechanistic details remain poorly understood for nucleocytoplasmic transport.
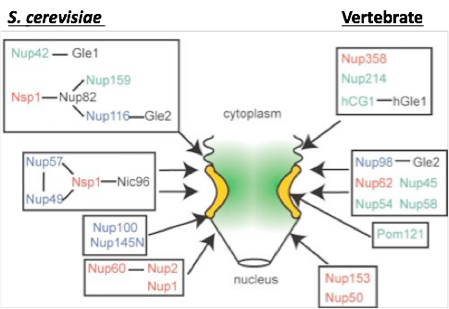
FG-Nups are distributed throughout the NPC. Nup subcomplexes and relative NPC substructural localization are shown with known subcomplexes boxed. Select structural, non-FG-Nups are in black text, while FG-Nups are denoted in color with respect to the FG repeat type: FG repeats = green text, GLFG repeats = blue text, FXFG repeats = red text.
As a model for understanding transport, we have explored mRNA export from the nucleus. The export of mRNA to the cytoplasm is essential for translation of the encoded protein, and the complexity of the mRNA cargo presents quite the challenge for the NPC. As it is synthesized, mRNA is packaged with proteins to form large messenger ribonucleoprotein particles (mRNPs). This protein composition is regulated and remodeled throughout the mRNP’s lifespan, with association and dissociation of specific proteins mediating each step. For an mRNP to be competent for export, it must have the correct complement of associated proteins, and we have shown that specific changes in mRNP composition can regulate export in response to cellular stress. For instance, during heat shock, most polyadenylated mRNAs are retained in the nucleus while only shock-induced mRNAs are exported. We are interested in determining the mechanisms controlling such selective modulation of mRNP composition and export. Recent studies in our laboratory suggest that heat shock stress induces changes in mRNP composition via protein phosphorylation and nuclear sequestration of specific mRNP components. To determine what other changes direct the selective export, we are investigating the protein composition of mRNPs and the subcellular distribution of transport factors under an array of conditions that perturb mRNA export.
A major step where the mRNP protein complement is remodeled is at the cytoplasmic face of the NPC. Here, the actions of the essential Dbp5 and Gle1 proteins remove transport-specific proteins bound to an mRNP and release it into the cytoplasm, thereby controlling the directionality of mRNA export.. Dbp5 is a DEAD-box protein belonging to the RNA helicase superfamily II that binds to the nucleoporin Nup159 on the cytoplasmic filaments of the NPC. Likewise, Gle1 binds Nup42, also at the cytoplasmic filaments, and functionally requires the cytoplasmic production and binding of inositol hexakisphosphate (IP6). Interestingly, Gle1 self associates at the nuclear pore complex, a function that is required for its role in mRNA export. The RNA-dependent ATPase activity of Dbp5 is specifically stimulated by IP6-bound Gle1, resulting in an ADP-bound form of Dbp5 that triggers remodeling of the mRNP. At this step, Nup159 acts as a nucleotide exchange factor to stimulate release of the bound ADP from Dbp5 after hydrolysis. Thus, Nup159 serves at least two key functions, to localize Dbp5 with Gle1 to remodel mRNPs and to recycle Dbp5 for additional rounds of mRNP export. This is the first known Dbp nucleotide release factor and may give insight to the regulation of other DEAD-box helicases that act on mRNPs. However, the story continues to evolve. Current studies of Nup159 and Nup42 have focused on their discrete abilities to bind the mRNA transport receptor Mex67 via their FG domains and also interact with Dbp5 and Gle1, respectively. We recently determined that these characteristics allow the Nup42 and Nup159 FG domains to efficiently target the mRNP directly to Gle1 and Dbp5 for mRNP remodeling at the NPC. Importantly, only certain FG domains with binding sites for Mex67 were functional at the NPC cytoplasmic face, and placement of the Nup42 FG domain at the site of remodeling by fusing it to Gle1 was sufficient for function. These studies provide key evidence that character and context do play a direct role in FG domain function and mRNA export, and set the stage for further discoveries regarding the fascinating specificity of function among FG-Nups.