The incidence of diabetes continues to increase worldwide. It was estimated that by the year 2030, 4.4% of the population will suffer from diabetes. The goal of this project is to understand the role of the microtubule (MT) cytoskeleton in the progression of diabetes and, in particular, in insulin secretion. Our unique approach combines the traditional mouse models of diabetes with modern imaging technique, such as super-resolution microscopy, total internal refection (TIRF) microscopy, and high-resolution confocal microscopy. With these powerful techniques, we have resolved the MT network organization in islet β-cells and have uncovered an unconventional MT-dependent regulation of insulin secretion. An increase of glucose induces MT disassembly, and therefore facilitates insulin secretion (Zhu et al. 2015). We have determined that MT-associated protein tau is a critical player is this regulation (Ho et al 2020). At the same time, glucose facilitates new MT nucleation at the Golgi via cAMP-dependent pathway, as characterized by Kathryn Trogden in the lab (Trogden et al 2019). MTs also regulate the localization and availability of IGs in beta cells (Bracey et al. 2020). Our next step is to determine the mechanistic details of MT-dependent regulation of secretion and to dissect its role in the development and treatment of diabetes in humans. This project is in collaboration with Guoqiang Gu’s lab at Vanderbilt University and William R. Holmes lab at University of Indiana.
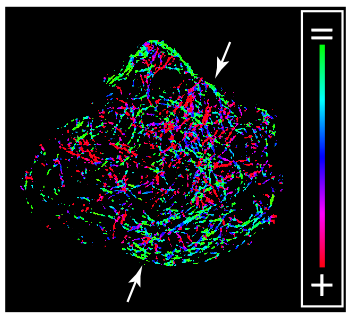
Confocal Z Stack Showing an Immunostained Primary Murine β Cell 90 s after Nocodazole Washout, Related to Figure 1E. The insulin is shown in blue and the tubulin is shown in green. The Golgi marker GM130 is shown in red and the white arrows are Golgi-derived MTs. (Zhu et al. 2015)
Understanding the role of α cells in promoting β cell MT turnover
Investigator: Kung-Hsien Ho
The cytoskeleton of pancreatic β cells plays important roles in regulating insulin secretion. Our labs previously showed that in addition to transporting insulin vesicles from the Golgi apparatus for secretion, microtubules facilitate the withdrawal of insulin vesicles from cell periphery to restrict basal insulin release. Increased glucose concentration induces microtubule disassembly in β cells to inhibit the withdrawal of insulin vesicles from periphery and enhances insulin secretion. My recent studies revealed that tau in β cells mediates the coupling of microtubule dynamics and glucose stimulation in a GSK3-depedent manner. Depletion of tau in mouse primary β cells leads to elevated basal insulin secretion, impaired stimulated secretion, and depletion of insulin content in the cytoplasm.
Pancreatic β-cells are highly specialized to meet the strong demand of insulin synthesis and secretion. Unlike many clonal cells, primary β-cells express a unique isoform of a well-studied microtubule minus end-binding protein, CAMSAP2, which has a microtubule-independent non-canonical function. In primary β-cells, CAMSAP2 localizes to the Golgi apparatus independent of microtubule-binding and promotes Golgi-ER trafficking to support efficient production of insulin vesicles. Knockdown of CAMSAP2 impairs Golgi-ER trafficking, reduces total insulin content and attenuates GSIS without affecting the stability and morphology of microtubules.
Kung-Hsien is currently studying the cross-talking between α and β cells and the potential mechanism of paracrine regulation of β cell microtubule dynamics to respond to glucose stimulation.
Relevant publications
1. Ho KH, Jayathilake A, etc. (2023) CAMSAP2 localizes to the Golgi in islet β-cells and facilitates Golgi-ER trafficking. iScience. 26(2):105938
2. Ho KH, Yang X, Osipovich AB, etc. (2020) Glucose regulates microtubule disassembly and the dose of insulin secretion via Tau phosphorylation. Diabetes 69(9):1936-1947.
3. Ho KH*, Bracey K*, etc. (2019) Microtubules regulate localization and availability of insulin granules in pancreatic β-cells. Biophys J. 118(1):193-206. (*equal contribution)
Determining the role of microtubule sliding in insulin secretion
Investigator: Kai Bracey
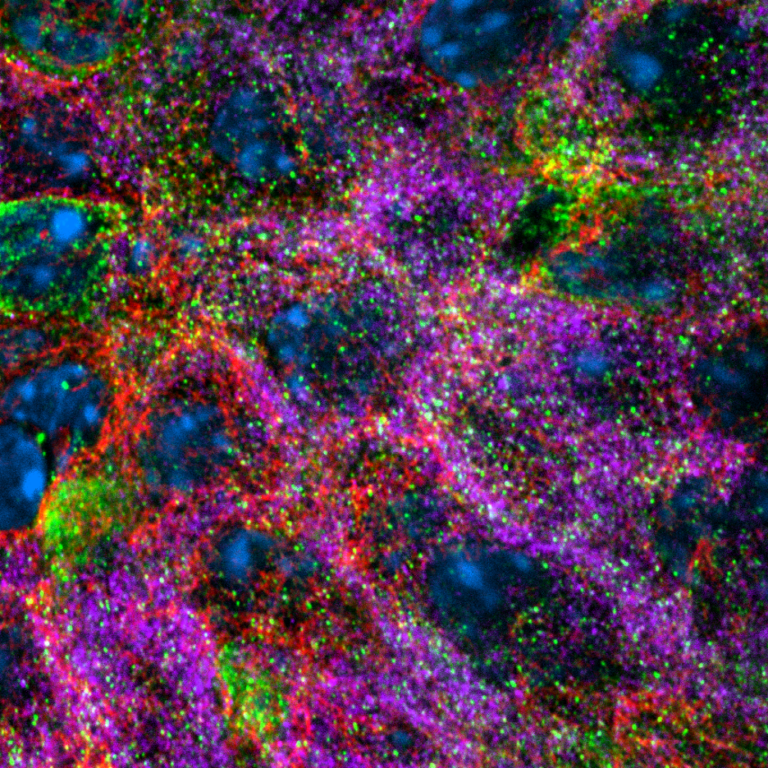
Beta cells need to secrete restricted doses of insulin, in order to reduce blood sugar to normal levels but not completely deplete it; this requires tight coordination between intracellular insulin storage and secretion. Preliminary data indicates that this coordination is regulated by microtubules and molecular motors that transport insulin granules to specific cellular locations. (schematic, right) Kai has found that glucose stimulation promotes a novel MT sliding, in which kinesins bind to MTs and use it as cargo, reconfiguring entire networks. Kai is studying the regulatory mechanism leading to MT sliding and the functional aspect of how sliding rates affect insulin granule trafficking.
Relevant publications:
Microtubules in Pancreatic β Cells: Convoluted Roadways Toward Precision
Microtubules Regulate Localization and Availability of Insulin Granules in Pancreatic Beta Cells.
MT (green) sliding demonstrated using photoconvertible probe (red)
Building computational model of molecular-motor dependent insulin granule transport
Investigator: Currently open
Delivering of secretory vesicles to correct destinations is a result of a collaboration of multiple molecular motors. Plus-end-directed motor kinesin-1 and minus-end-directed motor cytoplasmic dynein both transport insulin granules along MTs but how their functions are coordinated is unclear. This project aims to resolve functional interactions of beta-cell motors in experimental and computational spaces. We also seek to determine the consequences of integrated molecular motor actions for insulin granule positioning within the cellular space and for secretion responses.
Relevant publications:
Microtubules Regulate Localization and Availability of Insulin Granules in Pancreatic Beta Cells.