Inter-relationships & Functions of MT-binding Proteins
Microtubule (MT) plus-end tracking proteins (+TIPs) are a specialized group of proteins that preferentially localize to growing MT plus-ends. These include proteins such as End-binding proteins (EBs), CLIP-associated proteins (CLASPs), APC, MACF1 and many more. We have previously identified an essential role for CLASPs in non-centrosomal, Golgi-derived MT formation (Developmental Cell 2007). Ashley Grimaldi from our lab has shown that intricate relationships exist between the +TIPs. An example is how the localization EB1 on the plus-end is regulated by CLASPs (Developmental Cell 2014). This was the first example of another +TIP protein regulating the localization and dynamics of EBs at MTs.
We are interested in further dissecting functions and interactions of CLASP proteins in the cell.
Functions of CLASP paralogs at MT plus ends and the Golgi
Investigator: Alisa Cario
Human CLASPs consist of two paralogs: CLASP1 and CLASP2, which have been found associated with the cell cortex, kinetochores, the Golgi and at the ends of growing MTs. CLASPs are multifunctional proteins involved in MT dynamics, anchoring of MTs to diverse cellular structures, trafficking, etc. We aim to identify the functional significance of CLASP1 versus CLASP2 in specific functions. Our data suggest a novel role for CLASP2 in specific steps of post-Golgi secretory trafficking, while CLASP1 has a stronger role in MT dynamics control and collaboration with other MT-associated proteins.
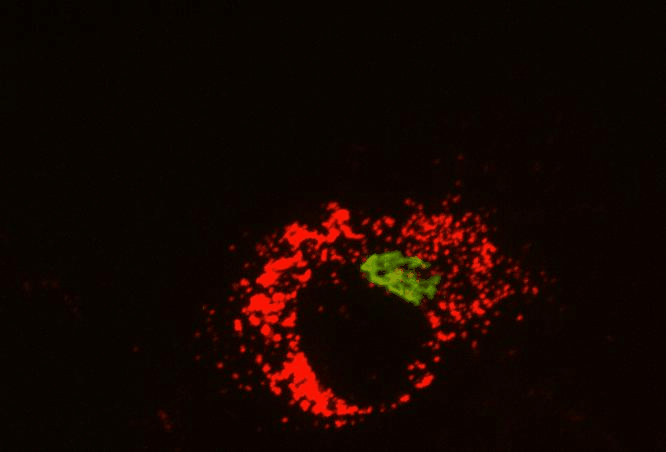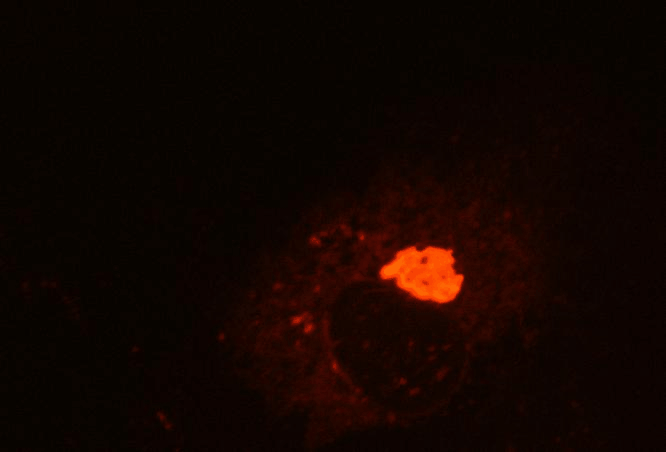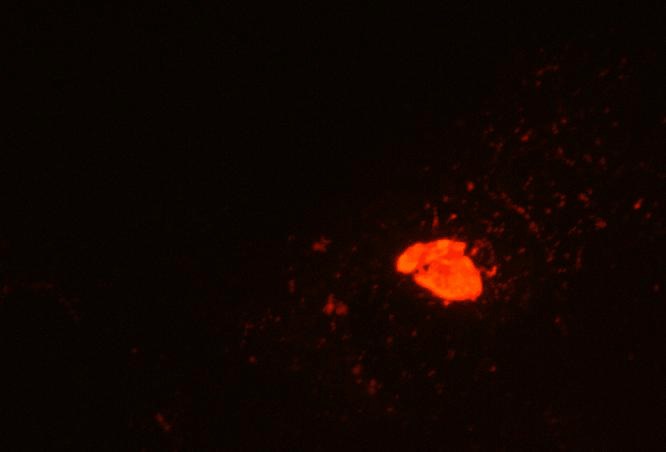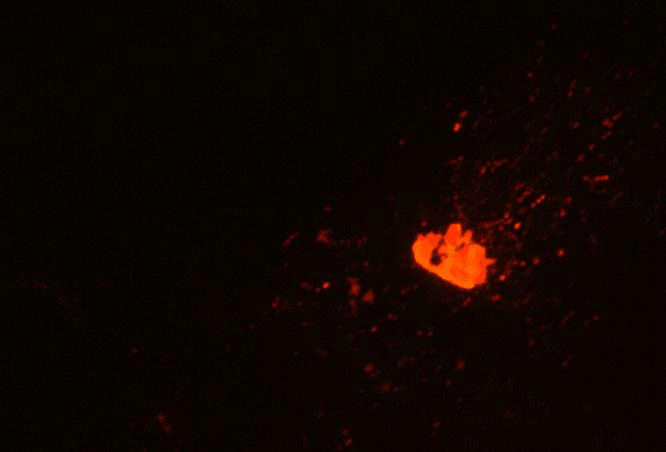
RASSF1A function in MT Network Dynamics and Golgi Positioning
Investigator: Currently open
Ras-association domain family 1A (RASSF1A) is a MT binding protein that is frequently silenced during cancer transformation. This loss of RASSF1A expression often correlates with increased levels of metastasis; however, the cell biological function of RASSF1A binding to MTs, or how loss of RASSF1A influences the MT network of interphase cells, has not been explored.
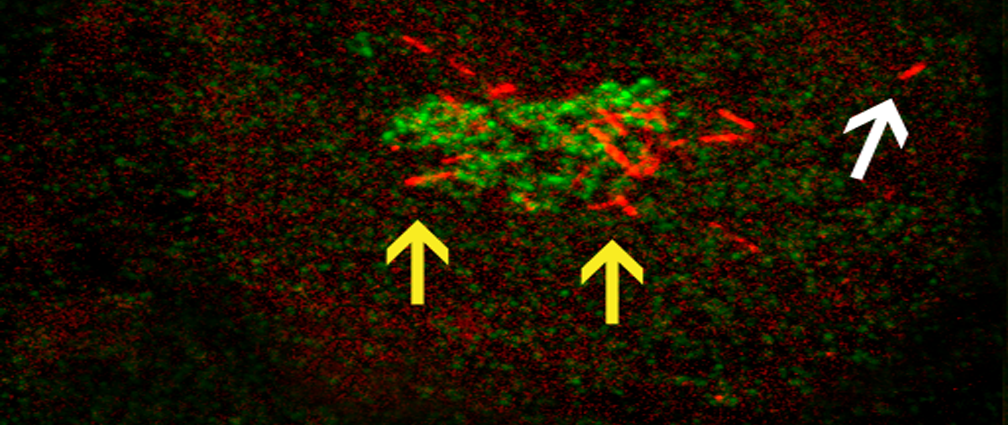
The goal of this project is to utilize a variety of high-resolution live-cell imaging techniques to examine the effect that RASSF1A has on MT network organization and dynamics as well as its influence on other organelles in the cell; in addition, we are examining how these functions of RASSF1A subsequently affect MT-based processes, such as cell migration and polarity. The picture shows two distinct localizations of the MT-binding protein RASSF1A: segmental RASSF1A localization to peripheral MTs (indicated by white arrow), and RASSF1A-bound Golgi-derived MTs (indicated by yellow arrows). The Golgi is shown in green, RFP-RASSF1A is shown in red.
Relevant publications: