Cytoskeleton at Insulin Secreting Adhesions
A recently described phenomenon in the field of islet research shows that pancreatic beta cells secrete insulin directly to the bloodstream, to optimize glucose clearance in tissues. It has also become clear that beta cells tend to cluster insulin secretion spatially, into secretion events often referred to as “hot spots,” in which there are several events next to each other. Our lab has shown that MTs regulate clustered insulin secretion at those hot spots (Trogden et al. 2021). These hot spots of directed secretion contain adhesion proteins, cytoskeletal components, and conserved neuronal active zone proteins, all of which are mechanically regulated. This has sparked our lab’s interest in characterizing these emerging structures as “insulin secreting adhesions” (ISAs) (Fye & Kaverina 2023), studying them by combining cutting-edge high-resolution microscopy, live exocytosis detection assays, and mechanobiology techniques.
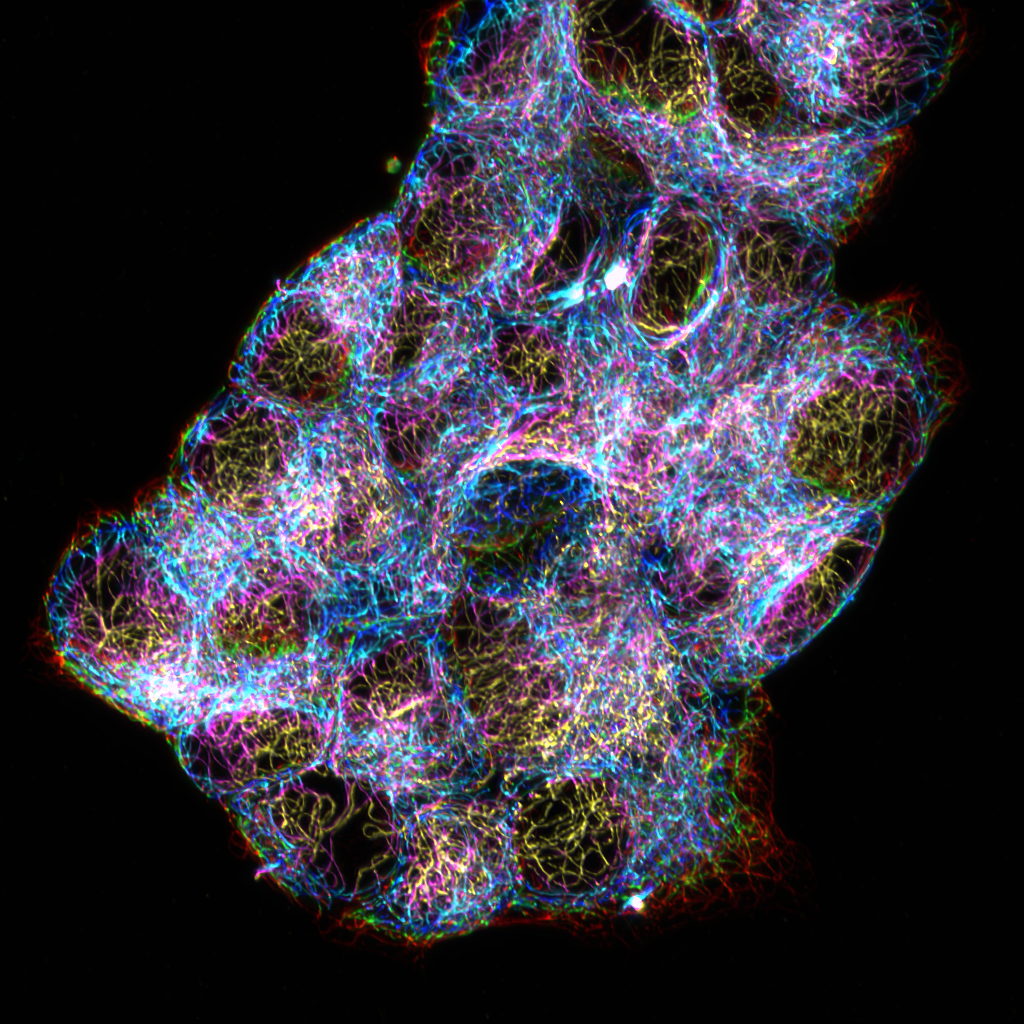
The role of the cytoskeleton at ISAs
Investigator: Maggie Fye
Our lab has previously shown that MT depolymerization increases both the number of active ISAs per cell and the number of secretion events at each ISA (Trogden et al. 2021), but the mechanism underlying this regulation is uncharacterized. Actin and actomyosin contractility also regulate insulin secretion, but whether this applies specifically to ISAs is unknown. Using TIRF microscopy and an insulin secretion detection assay, Maggie is studying the dynamics and interplay of cytoskeletal networks at ISAs and by what mechanism they regulate clustered secretion.
Relevant publications:
Insulin secretion hot spots in pancreatic β cells as secreting adhesions
Actin dynamics in whole mouse pancreatic islets
CLASP proteins and mechanobiology of ISAs
Investigator: currently open
Our lab has previously shown that MT configuration at the cell periphery defines whether insulin secretory granules are delivered to or withdrawn from the secretion sites (Bracey et al 2020). We aim to understand the mechanisms underlying ISA-specific MT morphology. It is known that MT-associated protein CLASP can bind an ISA components LL5β, promoting MT capture. We are growing interested in the idea of CLASP as an important molecular player at ISAs, potentially controlling glucose-dependent MT configuration at ISAs and therefore availability of insulin granules for clustered secretion.
Actin (cyan) and insulin secretion events (yellow) in whole mouse pancreatic islets