Arrestins in Cell Adhesion and Motility
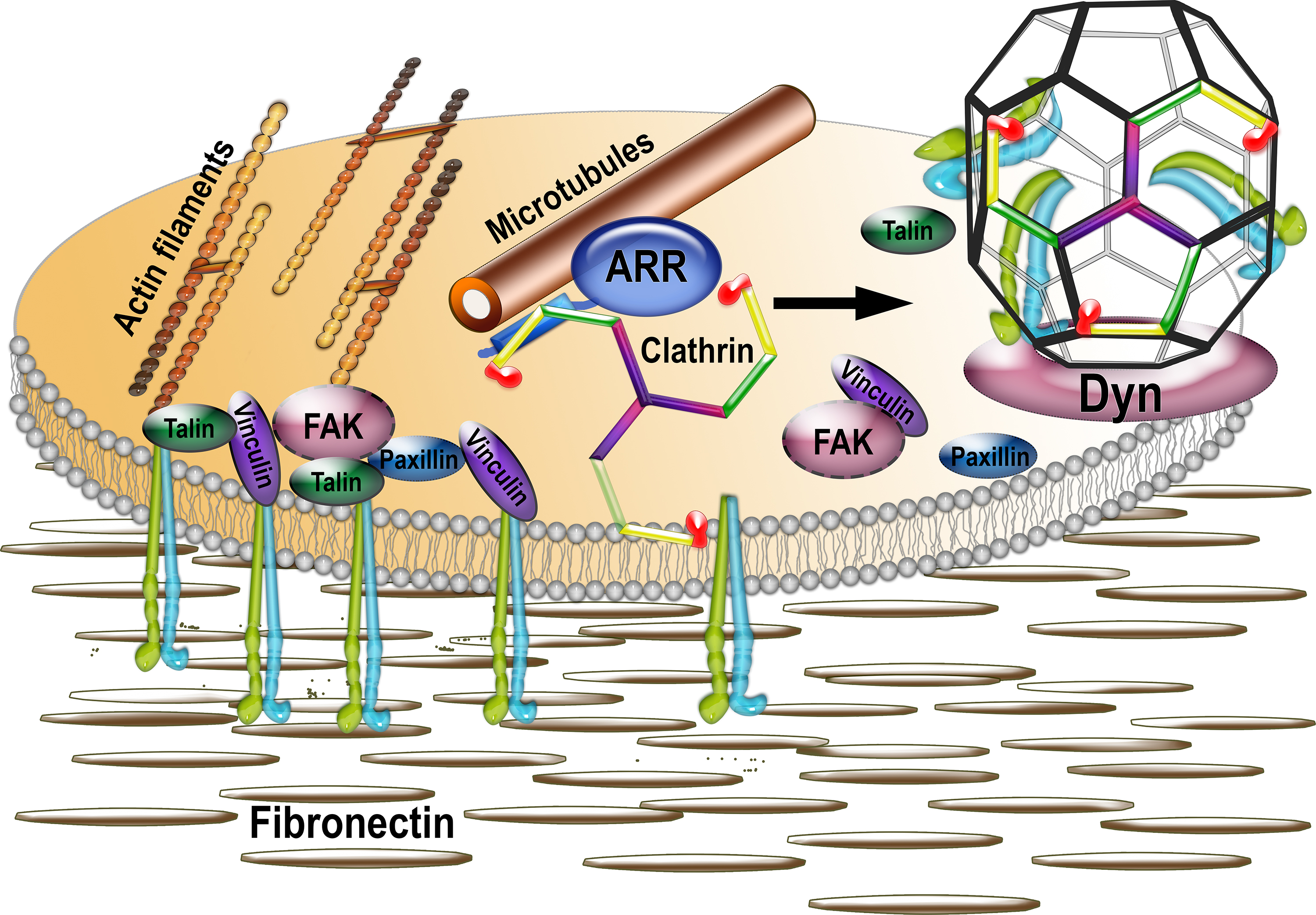
Focal adhesions are multi-protein complexes organized around clustered active integrins that bind extracellular matrix (shown as fibronectin). FAs are connected to actin filaments and include numerous structural and signaling proteins, such as talin, vinculin, paxillin, focal adhesion kinase (FAK), etc. FAs are very dynamic, and their disassembly is facilitated by the proximity of microtubules and triggered by clathrin-dependent internalization of integrins. Our data suggest that non-visual arrestins, known to interact with both microtubules and clathrin, serve as a link between the two, being delivered together with associated clathrin by microtubules to FAs. The delivery of arrestin-bound clathrin to FAs facilitates integrin internalization via clathrin-coated pits (with the help of dynamin, which pinches coated vesicles off of the membrane) and thus FA disassembly.
Arrestins are also important for regulating cell size, adhesion and motility. Focal adhesion (FA) play key role in cell attachment, and their timely disassembly is critical for cell motility. Both microtubule-dependent targeting and recruitment of clathrin are critical for FA disassembly. We have identified nonvisual arrestins as molecular links between microtubules and clathrin. Cells lacking both non-visual arrestins showed excessive spreading on fibronectin and poly-D-lysine, increased adhesion, and reduced motility. The absence of arrestins greatly increases the size and lifespan of FAs, indicating that arrestins are necessary for rapid FA turnover. Our data show that that arrestins are necessary for microtubule targeting-dependent FA disassembly. Clathrin exhibited decreased dynamics near FA in arrestin-deficient cells. In contrast to wild type arrestins, mutants deficient in clathrin binding did not rescue the phenotype. Collectively, the data indicate that arrestins are key regulators of FA disassembly that link microtubules and clathrin.
