Welcome to the Colbran Lab website
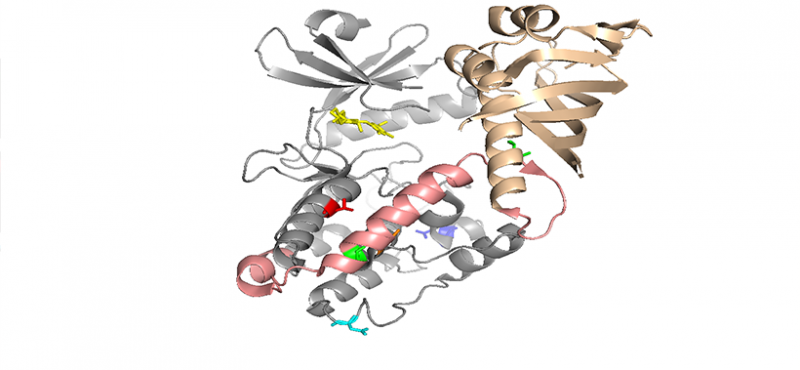
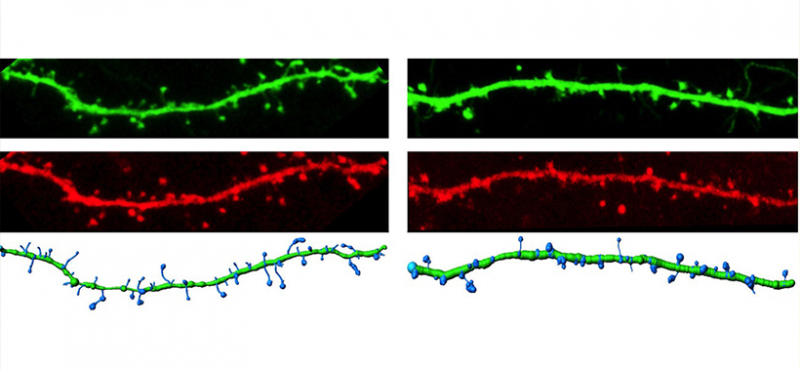
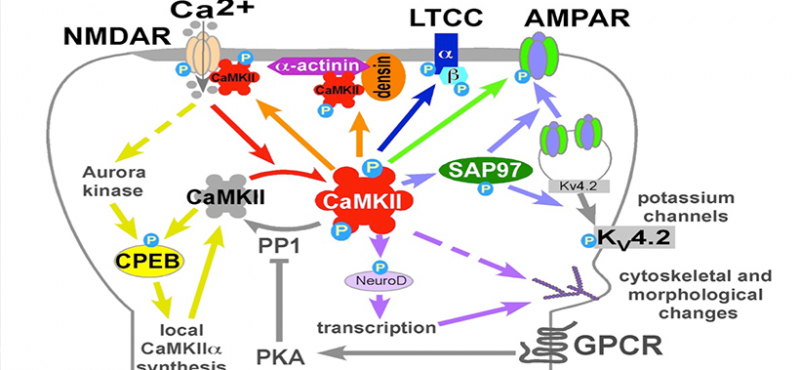
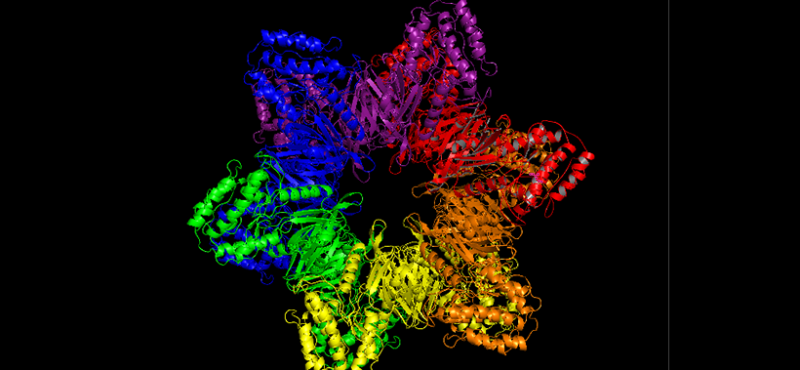
Our lab uses multidisciplinary approaches to investigate the physiological and pathological roles of calcium/calmodulin-dependent protein kinase II, with a focus on postsynaptic signaling at excitatory synapses. We are interested in the mechanisms underlying various forms of synaptic plasticity in multiple brain regions that are involved in different forms of learning and memory. In addition, our work is directed at uncovering derangements in these mechanisms that are associated with neuropsychiatric and neurological disorders.
Find our more about our research interests here
Our lab is in the Department of Molecular Physiology and Biophysics, one of the four basic science departments in the Vanderbilt University School of Medicine. We are also members of the Vanderbilt Brain Institute, The Vanderbilt Kennedy Center, The Vanderbilt Center for Addiction Research, The Vanderbilt Diabetes Research and Training Center and the Vanderbilt-Ingram Cancer Center.
- Cyclic AMP‐dependent protein kinase and D1 dopamine receptors regulate diacylglycerol lipase‐α and synaptic 2‐arachidonoyl glycerol signaling
- Neuronal L-Type Calcium Channel Signaling to the Nucleus Requires a Novel CaMKIIα-Shank3 Interaction
- CaMKIIα phosphorylation of Shank3 modulates ABI1-Shank3 interaction
- Activated CaMKIIα Binds to the mGlu5 Metabotropic Glutamate Receptor and Modulates Calcium Mobilization
- Role of striatal direct pathway 2-arachidonoylglycerol signaling in sociability and repetitive behavior
- A novel mechanism for Ca2+/calmodulin-dependent protein kinase II targeting to L-type Ca2+ channels that initiates long-range signaling to the nucleus
- Densin-180 Controls the Trafficking and Signaling of L-Type Voltage-Gated Cav1.2 Ca2+ Channels at Excitatory Synapses
- A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASD-Related Behaviors
- CaMKII-mediated phosphorylation of GluN2B regulates recombinant NMDA receptor currents in a chloride-dependent manner
- Thematic Minireview Series: Molecular Mechanisms of Synaptic Plasticity
- Differential CaMKII regulation by voltage-gated calcium channels in the striatum.
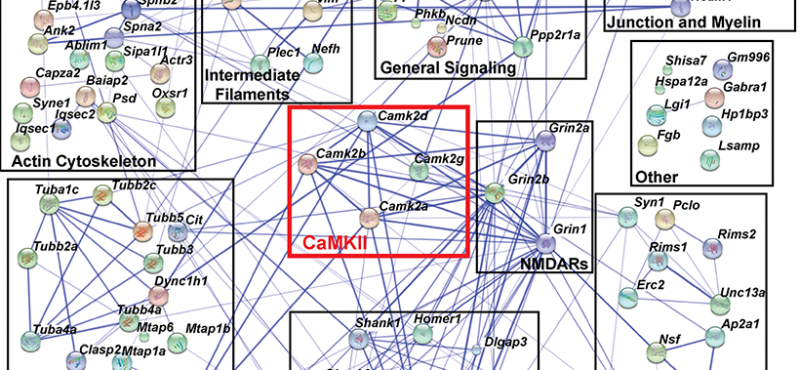
- Quantitative Proteomics Analysis of CaMKII Phosphorylation and the CaMKII Interactome in the Mouse Forebrain.
CaMKII, Calcium , Neuroscience , Biochemistry , Synaptic Plasticity , Long-Term Potentiation , Striatum , Behavioral Neuroscience, NMDA Receptors, AMPA Receptors, Voltage-Gated Calcium Channels , Endocannabinoids , Electrophysiology , Signal Transduction , Protein Phosphorylation , Phosphatases , Cardiomyocytes