RESEARCH
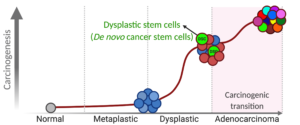
The Choi lab seeks to elucidate the biological mechanisms of cell lineage conversion and evolution during the malignant transformation of pre-cancerous cells. Epithelial cancers in the gastrointestinal tract most commonly develop within a carcinogenic cascade from pre-cancerous metaplasia to dysplasia and then adenocarcinoma. However, it is hard to clearly define stem cell hierarchy or functions in adenocarcinoma due to complex heterogeneity and diverse stem cell populations. The Choi lab has reconceptualized de novo phenomena and early features of carcinogenesis by defining dysplastic cells as epithelial cancer-initiating cells. I have pioneered a unique model design both in vitro and in vivo, to study pre-cancerous cell biology. This methodological advancement has provided us with opportunities to understand dynamic cellular and clonal activities in pre-cancerous cell lineage evolution at single cell level.
We are currently focusing on determining the molecular hierarchy based on metabolic, transcriptomic, and genetic status determining cell fates in pre-cancerous cells during carcinogenesis. We are also interested in stem cell functions during the regeneration of epithelial mucosa with damage or pre-cancerous lesions.
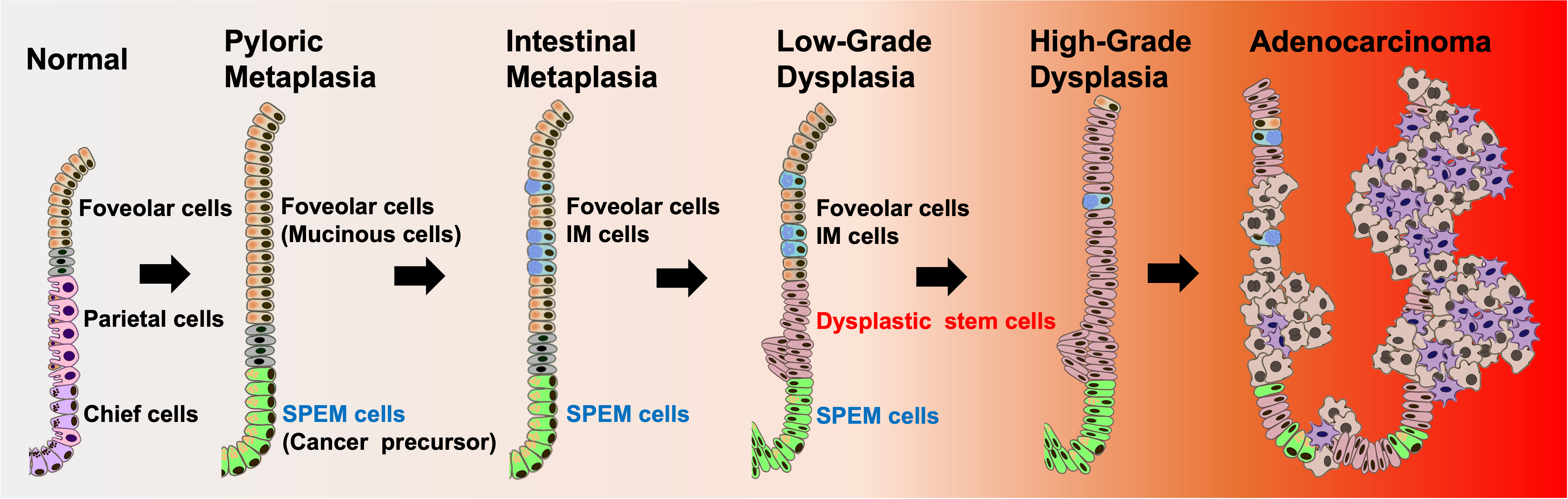
The Choi lab has recently established various pre-cancer organoid lines which recapitulate the phenotypes and characteristics observed in the gastric carcinogenesis cascade, as observed in mouse models and in human gastric cancer. The organoid lines display distinguishable structures and dynamic behaviors, pathological phenotypes and molecular signatures. The Choi lab investigated cellular heterogeneity and dynamics of dysplastic cell lineages as gastric cancer initiating cells using the dysplastic organoids and also identified dysplastic stem cell populations which are possible candidates for residual stem cell populations which can drive gastric cancer. The Choi lab has also developed several driver mouse models for targeted induction of responder genes only in zymogen granule-secreting chief cells which will allow us to overcome the limitations in present gastric cancer studies and have established several transgenic mouse alleles for understanding the full spectrum of gastric carcinogenesis.