Valvulogenesis
Valves of the Heart
The four-chambered vertebrate heart consists of aortic and pulmonic semilunar (SL) valves, as well as mitral and tricuspid valves separating the atria and ventricles. The coordinated opening and closing of the heart valves is required for unidirectional blood flow. The three cusps of each SL valve and the two (mitral) or three (tricuspid) leaflets of the atrioventricular (AV) valves is constituted by a complex stratified connective tissue.
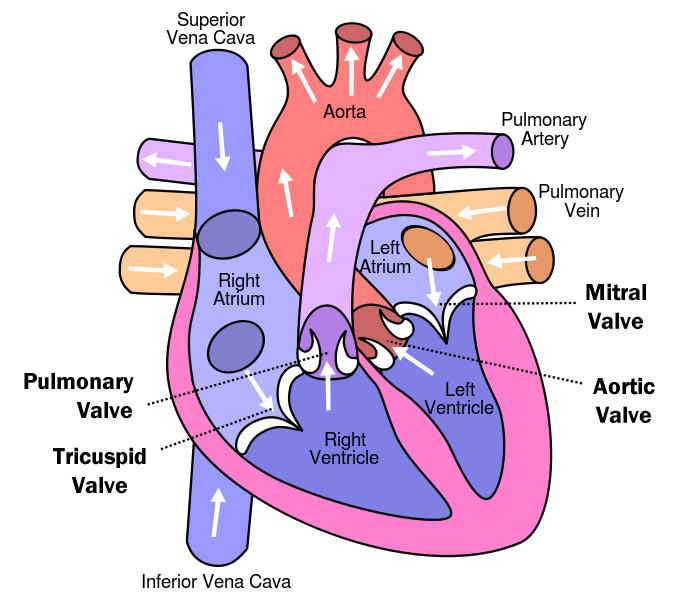
Figure 1. Layout and architecture of the human heart. Image acquired https://www.healthtap.com/topics/can-people-with-bicuspid-aortic-valve-live-long
Tricuspid Valve
Closes off the upper right chamber (or atrium) that holds blood coming in from the body.
Opens to allow blood to flow from the top right chamber to the lower right chamber (or from right atrium to right ventricle).
Prevents the back flow of blood from the ventricle to the atrium when blood is pumped out of the ventricle.
Related valve problems include: Tricuspid atresia, Tricuspid regurgitation, Tricuspid stenosis
Pulmonic Valve
Closes off the lower right chamber (or right ventricle).
Opens to allow blood to be pumped from the heart to the lungs (through the pulmonary artery) where it will receive oxygen.
Related valve problems include: Pulmonary valve stenosis, Pulmonary valve regurgitation
Mitral Valve
Closes off the upper left chamber (or left atrium) collecting the oxygen-rich blood coming in from the lungs.
Opens to allow blood to pass from the upper left side to the lower left side (or from the left atrium to the left ventricle).
Related valve problems include: Mitral valve prolapse, Mitral valve regurgitation, Mitral valve stenosis
Aortic Valve
Closes off the lower left chamber that holds the oxygen-rich blood before it is pumped out to the body.
Opens to allow blood to leave the heart (from the left ventricle to the aorta and on to the body).
Valvulogenesis during embryonic development begins with the formation of endocardial cushions in the AV canal (AVC) and outflow tract (OFT) of the primitive looped heart tube (See Human Embryology) . From here the valve primordia that coincides with individual leaflets and cusps are derived from the endocardial cushions.
- The precise cushion origins of specific valve components are not well-defined.
For the AV valves, the septal valve leaflets are derived from the fused inferior and superior endocardial cushions that form in the AVC of the primitive heart tube. Mural leaflets are derived from mesenchymal cushions that arise laterally in the AVC after cushion fusion.
Less is known of how the SL valves arise from the complex arrangement of proximal and distal cushions that form in the OFT.
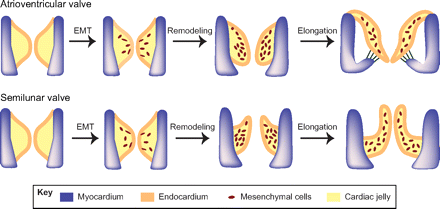
Figure 2. Epithelial-to-mesenchymal transformation and valve elongation. Endocardial cells in the AV cushions and conal cushions undergo epithelial-to-mesenchymal transformation (EMT) and generate mesenchymal cells that populate the cushions. The mesenchymal cushions then remodel and elongate themselves to from primitive valves that mature into thin valve leaflets. Image acquired from Partitioning the heart: mechanisms of cardiac septation and valve development
The valve progenitor cells of the endocardial cushions are highly proliferative, whereas little or no cell cycling is apparent later in remodeling and mature valves. The valve primordia will grow and extend to form thin fibrous leaflets of the AV valves and cusps of the SL valves, with increased ECM deposition and remodeling. During late gestation and soon after birth, the valve leaflets become stratified into highly organized collagen-, proteoglycan-and elastin-rich ECM compartments resulting in the specified valve.