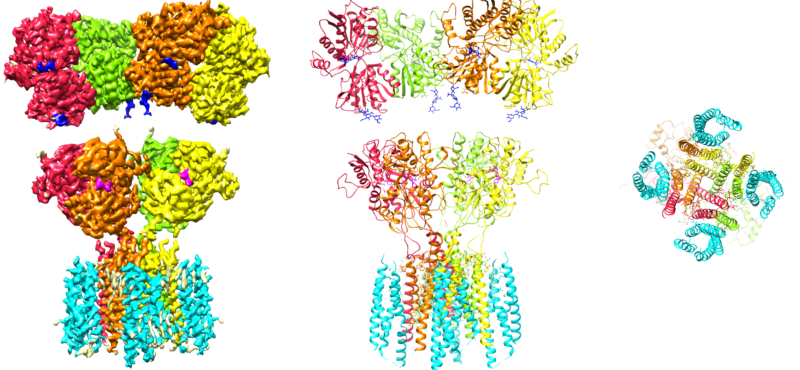
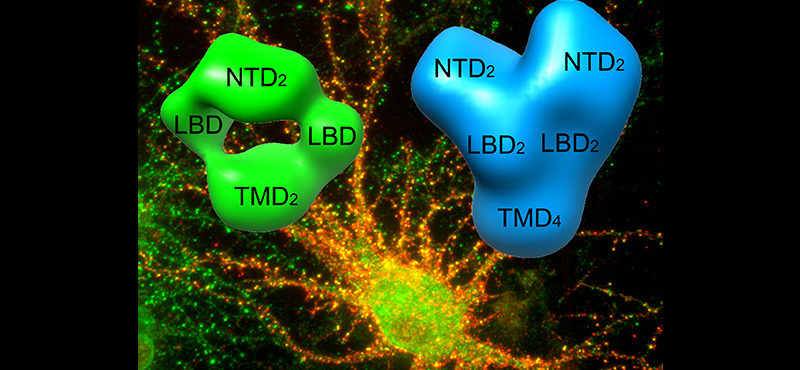
Welcome
Laboratory for investigating molecular mechanism of synaptic modulation and plasticity
- Signaling mediated by the ionotropic glutamate receptors
- Structure based therapeutics discovery
- Biophysics of learning and memory
Interested Diseases: autism, schizophrenia, Alzheimer’s disease, ALS, intellectual disability, brain tumor, seizure, limbic encephalitis, and neurodegenerative disorders.