Research
To understand the mechanisms of receptor mediated signaling, the Mudumbi lab combines cutting-edge live cell imaging techniques – single-particle tracking (SPT), single-molecule Förster resonance energy transfer (smFRET), super-resolution microscopy, and single-cell FRET to name a few – with a number of other biochemical and molecular biology approaches (phosphoproteomics, signaling assays, etc). The research in the Mudumbi lab has two main focuses: 1) receptor activation kinetics and 2) downstream signaling kinetics. By understanding the kinetics of signal transduction from the level of both receptor signaling and downstream events, we hope to build a ‘kinetic landscape’ to understand how receptors achieve distinct signaling responses through the same receptor. This will allow us to target these kinetics with therapeutics and modulate or ‘fine-tune’ them for specific cellular outcomes.
Receptor Activation Kinetics
Several receptor tyrosine kinases (RTKs) have multiple different activating ligands that can elicit distinct signaling outcomes by activating the same receptor, a phenomenon known as biased signaling. A prime example of this is the epidermal growth factor receptor (EGFR), an RTK in the ErbB family of receptors, which has 7 different activating ligands (EGF, TGFα, BTC, HB-EGF, EREG, EPGN, and AREG). Work from several labs (Freed Cell 2017, Kim Cell 2017, Ho Cell 2017, Huang Cell Rep. 2017) has started to suggest that receptor activation kinetics likely controls the biased signaling exhibited by these receptors, and that mutations can alter both a receptor’s affinity for different ligands as well as its dimerization kinetics. The Mudumbi lab has developed tools to directly observe and quantitate the dimerization kinetics of receptors in living cells. Focusing on the ErbB family of receptors, we are interested in studying receptor dimerization kinetics, and how these kinetics are altered by different activating ligands and mutations. We are also very interested in studying how dimerization kinetics are altered to modulate signaling by heterodimerization.
Another focus of the lab is on the kinetics of receptor organization on the membrane and the trafficking of receptors to and from the plasma membrane.
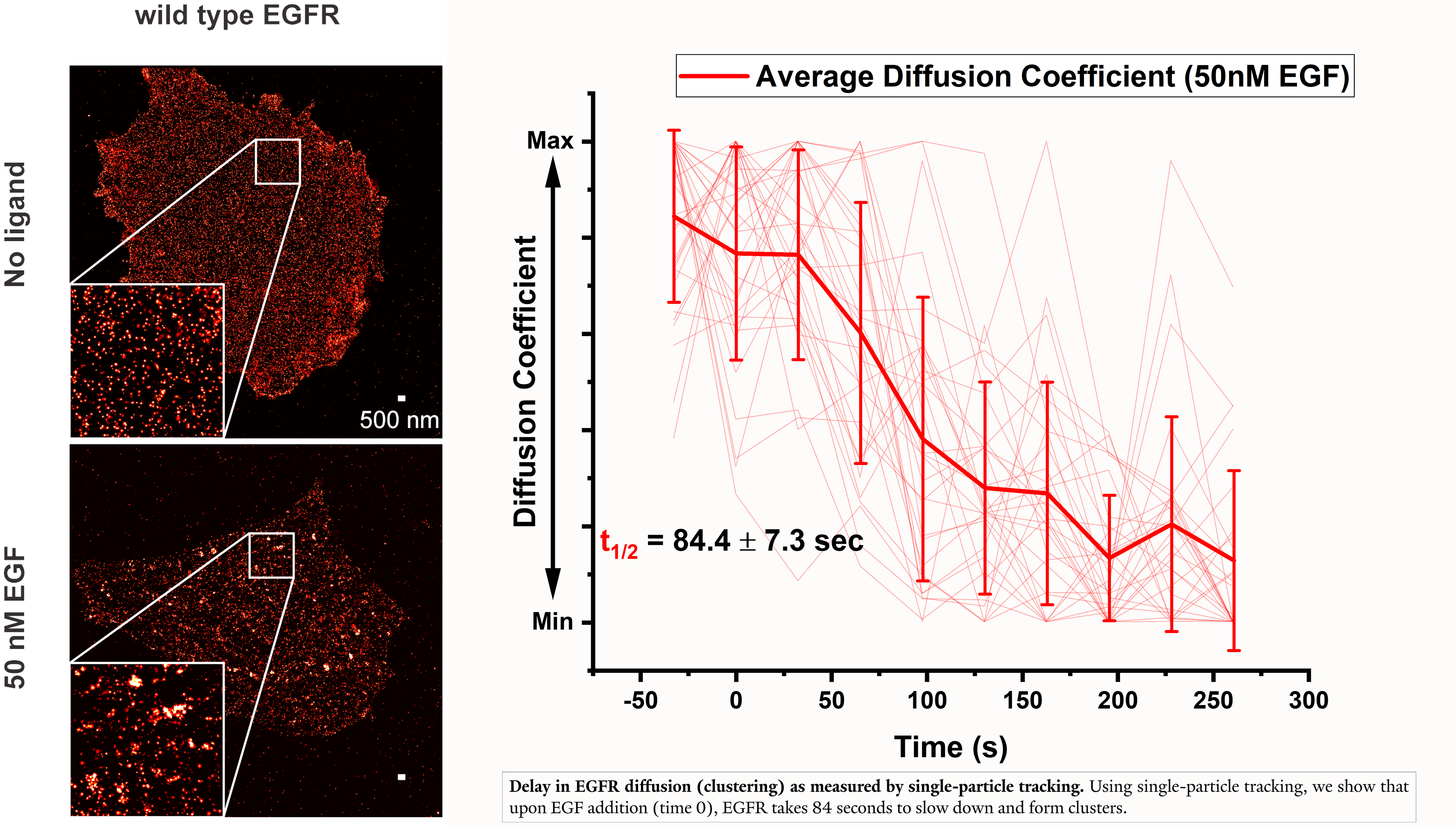
Downstream signaling kinetics
It is well known that many RTKs are phosphorylated intracellularly upon activation, and that the resulting phosphotyrosines recruit various downstream signaling molecules and adaptors. The kinetics of phosphorylation remain a mystery, however, and there is very little information on any ‘order’ of phosphorylation or the kinetics of adaptor/signaling protein recruitment and activation. Using a variety of approaches, the Mudumbi lab is asking questions such as: what is the duration of phosphorylation at different tyrosine residues? What is the turnover rate of adaptor proteins? What is the stoichiometry of adaptor protein recruitment?