Directed Evolution of Drugs: RiPP Display
Due to the successes of therapeutics like the GLP-1 agonists, peptide drugs have recently begun to gain popularity with pharmaceutical companies, with an average of one a year now being approved by the FDA. Specifically, macrocyclic peptide compounds have become especially prevalent in drug development because of their favorable pharmaceutical properties, including improved cell permeability, metabolic stability, and a lower entropic binding penalty relative to their linear counterparts. The pharmaceutical industry has used these characteristics to design macrocyclic peptide drugs that treat cancer, viral infections, and bacterial infections. Despite these early successes, there remains a vast, undrugged biological space to explore.
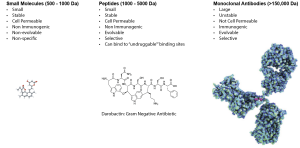
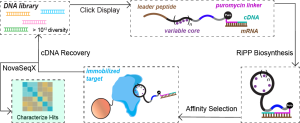
RiPPs are a diverse group of natural products that have been observed to have unique pharmacological properties, including antibacterial, anticancer, and antibacterial activities. Through their novel enzyme chemistry to form uniquely constrained macrocycles, RiPPs offer enormous untapped wealth of therapeutically-privileged scaffolds to design the next generation of peptide therapeutics. The rising popularity of peptide therapeutics has led us to direct our knowledge about RiPP pathways towards engineering new peptide therapeutic leads. These efforts are based on utilizing mRNA display technology to screen millions of substrate sequences to discover new high-affinity binders of valuable therapeutic targets. In mRNA display technology, the translated peptide is linked to its encoding mRNA sequence enabling the recovery/identification of the peptide binders through sequencing upon panning the displayed libraries against the target of interest. This technology can also probe the substrate scope of RiPP biosynthetic enzymes, providing massive datasets that shine light on how these enzymes perform their unique chemistry. Our lab is in the early stages of harnessing this library display technology to identify new RiPP-derived therapeutic leads to help fill this gap and begin treating untreated or poorly treated diseases.