Research Program
Research in the Coate laboratory seeks to define the cellular and genetic mechanisms that regulate the specialized identity and physiological function of human pancreatic islet endocrine cells in health and disease.
The physiological, cellular, and molecular mechanisms that govern the function and maintenance of human pancreatic islet endocrine cells in healthy individuals remain poorly defined. Furthermore, we do not know what causes these cells to become dysfunctional during the development of metabolic diseases such as type 2 diabetes (T2D). The last decade has witnessed not only improved access to human pancreatic tissue but also dramatic expansion in the number of tools that enable interrogation of cell-specific features with unprecedented molecular, spatial and temporal resolution. Our goal is to harness these tools to make discoveries that advance our knowledge and understanding of human pancreatic islet biology.
Our research is guided by the following questions: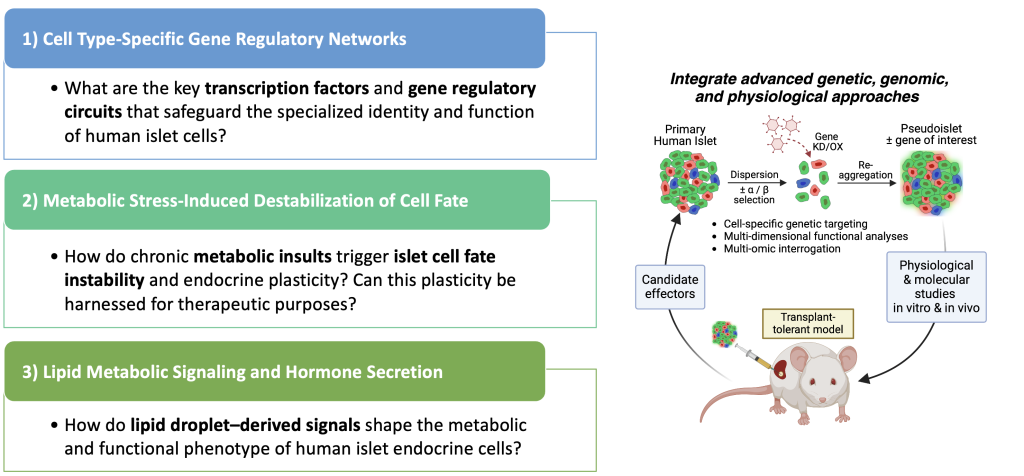
Current projects in the lab include:
- Defining how islet-enriched transcription factors (TFs) regulate the activity and identity of adult human alpha and beta cells. Studies principally performed in mice have established critical roles for pancreatic islet-enriched TFs in alpha and beta cell development and function. Strikingly, most (if not all) forms of diabetes are associated with islet-enriched TF inactivity. We are investigating the role of one such TF, MAFB, which we have found to be an essential regulator of the molecular and functional identity of human alpha cells. Analogous studies are also underway for ISL1, another islet TF linked to obesity and T2D. For both MAFB and ISL1, studies are underway to 1) Map their cell-specific gene regulatory circuits in human alpha and beta cells, 2) Clarify the interplay between these and other transcription factors in maintaining islet cell identity, 3) Assess the functional consequences of compromised MAFB and ISL1 activity in transplanted human islets in vivo.
- Determining how lipid droplets (LDs) and LD-derived signals impact the metabolic and functional properties of human alpha and beta cells under normal and pathological conditions. LDs are dynamic intracellular organelles that affect many aspects of cell physiology and adaptively increase in response to cellular stress. LDs uniquely accumulate in human (as opposed to rodent) pancreatic islets in an age- and disease-dependent manner and appear to be critical in maintaining human beta cell function and health. We have identified a regulatory role for LDs and specific lipid mediators in controlling the physiological function of human alpha and beta cells. Studies are underway to test the hypothesis that LD accumulation is part of the adaptive response to metabolic stress that protects human islet cells challenged by glucose and/or lipid-induced toxicity.