Research
Our laboratory is dedicated to investigating the fundamental differences between healthy cells and cancer cells. Due to the high proliferative pressure, cancer cells experience various types of stress, and, to withstand this stress, they evolve and utilize several unique molecular pathways. Our primary research objective is to gain a mechanistic understanding of these pathways, with the intention of specifically targeting them to challenge cancer cells while minimizing effects on normal cells.
To achieve our goals, we will employ genomics, proteomics, and genetic approaches, as well as screen small molecule drug libraries and whole genome CRISPR libraries. This broad spectrum of approaches offers great potential for discoveries, simultaneously cultivating an ideal learning setting for both graduate students and post-doctoral fellows. Currently, our laboratory is focused on two primary research areas.
MiDAS
Duplication of the entire genome during the synthesis phase (S phase) of the cell cycle by the process of DNA replication is fundamental for the viability of all cells. Any perturbation to the DNA replication process, caused by physiological or pathological conditions, is called replication stress. Replication stress is characterized by slower or perturbed progression of the replication machinery and is a major cause of genome instability in cancer cells. In fact, replication stress is an emerging hallmark of cancer. Cancer cells experience high replication stress due to their intense proliferative pressure. As a result, they often fail to completely replicate their genome during S phase, particularly at difficult-to-replicate regions of the genome such as telomeres and fragile sites.
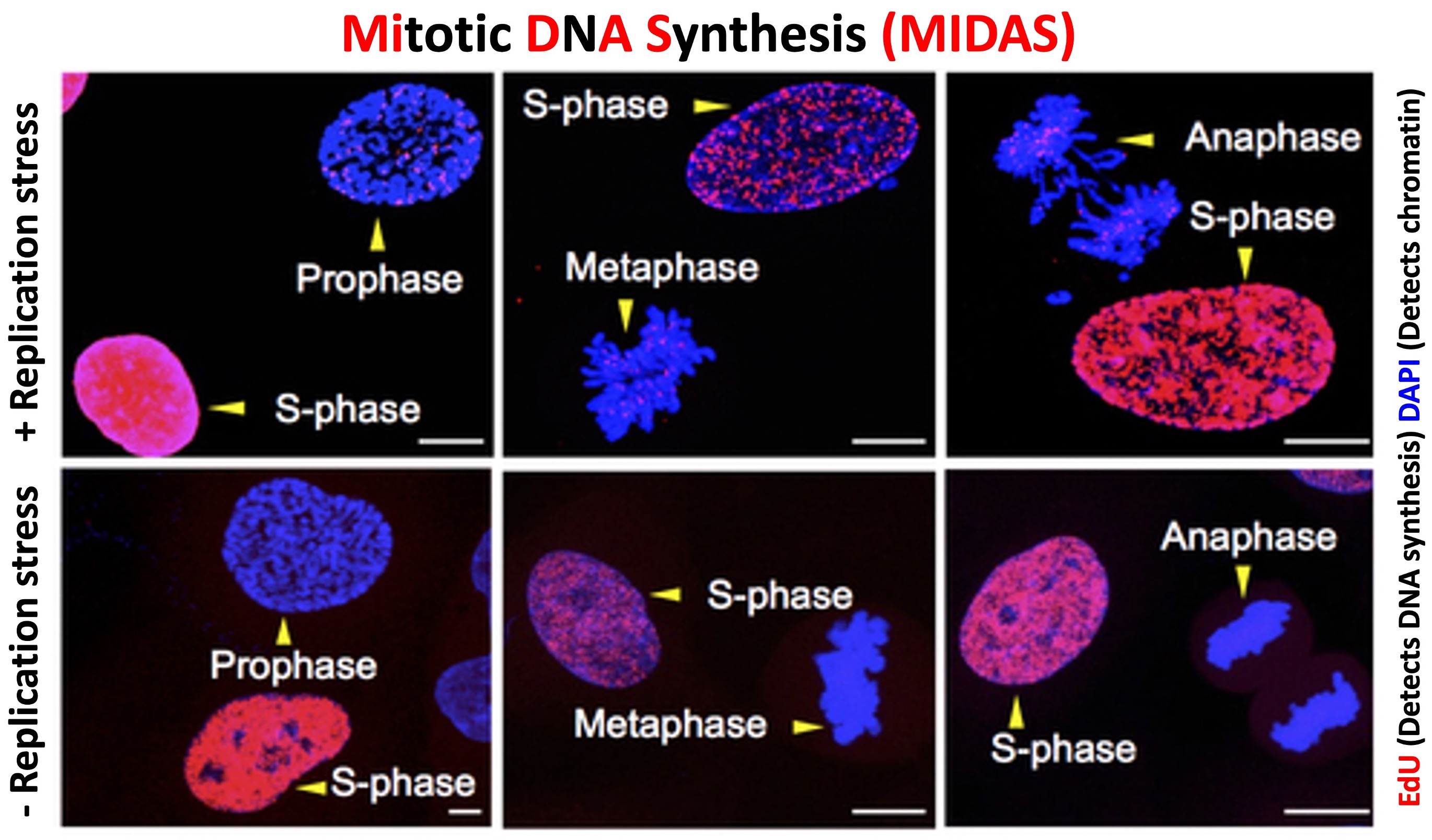
To overcome these replication challenges, cancer cells rely on Mitotic DNA Synthesis (MiDAS), a unique pathway that enables them to entirely duplicate their genome. MiDAS is a salvage DNA synthesis pathway, that assists cancer cells in maintaining genome stability by initiating DNA synthesis beyond S phase, particularly in mitosis. Almost all cancer cell lines derived from human cancers exhibit MiDAS. However, normal healthy cells barely carry out MiDAS because of their active cell cycle checkpoints and lack of intrinsic replication stress. This nearly exclusive utilization of MiDAS by cancer cells, makes it a promising therapeutic target. Our long-term goal is to mechanistically understand this pathway and explore its therapeutic aspects by identifying inhibitors of the MiDAS pathway.
Transcription-replication dynamics
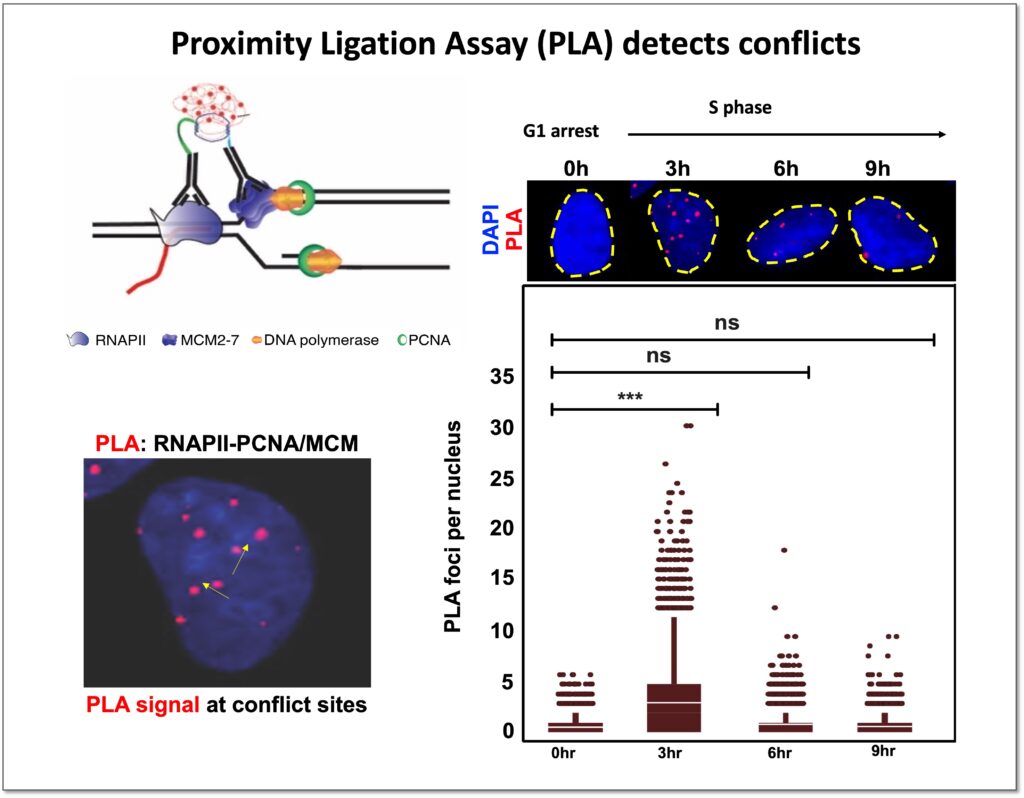
Coordination between the DNA replication and transcription processes is one of the major challenges that cells face during proliferation. Since both replication and transcription machineries use the same genomic DNA as a template, spatial-temporal separation between these two processes is necessary to avoid conflicts. Cells have evolved different mechanisms to evade conflicts, but physiological contexts such as the proximity of transcription start site to replication origins, or diverse transcription programs throughout different cell types combined with the vast number of replication origins in human cells makes distinct demarcations between these two processes hard to achieve.
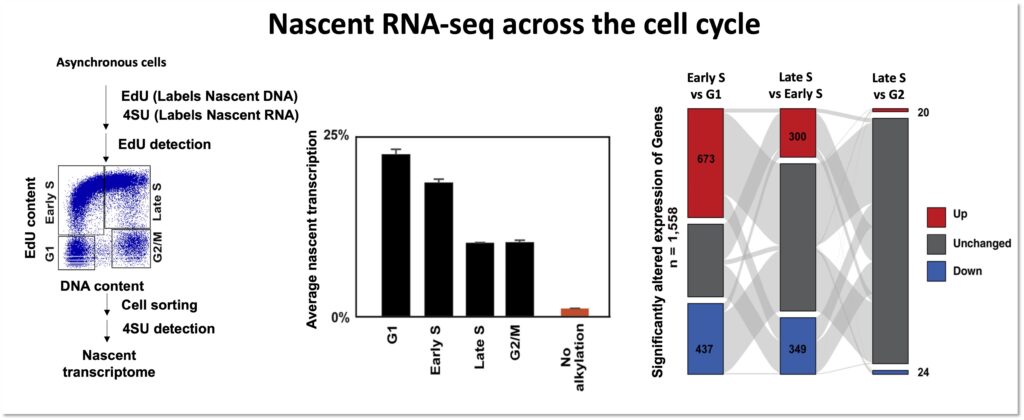
Moreover, several pathological conditions, such as oncogene activation during cancer onset, further complicate matters by altering replication and transcription programs and inducing transcription-replication conflicts. Transcription-replication conflicts trigger genome instability by disrupting DNA replication process, and thus play a crucial role in tumorigenesis. Our ultimate objective is to improve our understanding of the dynamics between transcription and replication in healthy cells and identify how it differs in cancer cells. This will not just enhance our knowledge of transcription-replication coordination during tumorigenesis, but also establish new approaches for targeted cancer treatments.