Assembly of the division apparatus
Eukaryotic cells accomplish cell division with exquisite spatial and temporal control. A key event of cytokinesis is the reorganization of the actin cytoskeleton into a contractile ring (CR). CR formation and most of the proteins contributing to this complex structure are conserved from yeast to humans, making the fission yeast S. pombe a powerful model organism for studying cytokinesis. Additionally, we employ a powerful combination of experimental techniques including sophisticated super-resolution imaging, biochemical reconstitution, mass spectrometry, and genetics to understand this phenomenon.
Our research into the CR apparatus centers on 4 broad categories. Click on the links to jump ahead to each category. Also check out our recent review on contractile ring function (Mangione et al., 2019).
I. Formin Cdc12, the formin necessary for assembling the CR actin
II. F-BAR proteins, part of the glue that links the CR actin to the plasma membrane
III. Spatial relationship of CR components
IV. The role of phosphoinositides in anchoring the CR to the plasma membrane
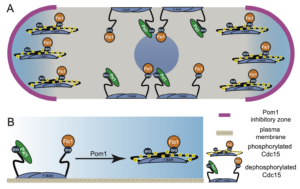
Models of Pom1 inhibition of Cdc15 function.
A) Model of Pom1 inhibition of Cdc15 membrane binding at cell tips in a single interphase cell with the nucleus in the middle.
B) Molecular model of Pom1 inhibition of Cdc15 membrane binding.
III. Spatial relationship of CR components
We are interested in using the fission yeast CR as an example of how the division site is organized in a eukaryotic cell. As described, we’ve discovered that Cdc15 forms a multivalent scaffold on the plasma membrane while simultaneously interacting with numerous proteins through both its N-terminal F-BAR domain and C-terminal SH3 domain. However, we do not know how all of these proteins are spatially organized relative to the membrane, CR actin, and each other. To obtain an interaction map of cytokinetic ring components, we employed a combination of super-resolution fPALM microscopy and intermolecular FRET and found that the fully formed and unconstructed CR is stratified into three layers; a membrane proximal layer, an intermediate layer and a distal layer. The membrane proximal layer consists of membrane scaffolds such as the F-BAR Cdc15 and anillin-like Mid1. The intermediate layer consists of many signaling proteins while the distal layer contains F-actin and actin binding proteins (McDonald et al, 2017). We are interested in better understanding how proteins may rearrange upon constriction of the contractile ring to understand how this process is initiated and completed.
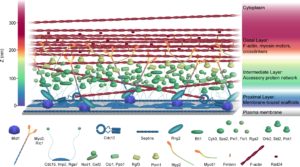
Figure from McDonald et al, 2017. Available at https://elifesciences.org/articles/28865 under the terms of the Creative Commons Attribution License.
Another way we are discovering how components of the CR are spatially organized is by reconstituting the cytokinetic machinery in vitro on synthetic membranes that allow imaging of labeled components by total internal reflection fluorescence (TIRF) microscopy. This system allows us to ask if/how proximity to membranes influences the molecular activity of major CR components. The results of this work will provide further information about the organization of molecules at the division site and how spatial arrangement might restrict or promote individual molecular functions.
IV. Plasma membrane lipid composition
The CR is assembled at the plasma membrane, which is composed of a phospholipid bilayer interspersed with proteins and cholesterol. The fission yeast gene efr3 encodes a component of the plasma membrane phosphoinositide (PI) 4-kinase complex that produces PI4P. Deletion of efr3 (efr3∆) results in cytokinetic defects (Chen et al., 2015). Our recent studies revealed that plasma membrane phosphoinositide lipid composition is altered in efr3∆ and this results in CR anchoring defects (Snider et al., 2017). In other words, CRs slide from the cell center in efr3∆ cells. Following up on this work we found that a mutation in the PI-5-kinase Its3 also has CR anchoring defects, implicating PI(4,5)P2 levels in maintaining at stable medially anchored contractile ring (Snider et al., 2018). We are taking advantage of these strains to determine the role of phosphoinositides in cytokinesis and to identify the full range of mechanisms and proteins that anchor the CR to the plasma membrane.